CHAPTER 22 Other Primary Tumors of the Lung
CARCINOID TUMORS
Approximately 25% of carcinoid tumors are bronchopulmonary (BP).1 The Surveillance, Epidemiology, and End Results (SEER) database shows that BP carcinoid tumors represent approximately 1.2% of primary lung malignancies.2 The current BP neuroendocrine tumor classification is based on 2004 World Health Organization (WHO) guidelines and describes four histologic subtypes of BP neuroendocrine tumors: typical carcinoid tumor (low grade), atypical carcinoid tumor (intermediate grade), large-cell neuroendocrine carcinoma (high grade), and small cell lung carcinoma (high grade).3
BP carcinoid tumors occur almost equally in men and women; however, in patients less than 50 years of age, they are observed nearly twice as often in women.4 Modlin and colleagues conducted a 5-decade meta-analysis of carcinoid tumors, which demonstrated that BP carcinoid tumors were more common in whites (black-to-white ratio, 0.45), Asians (Asians-to-non-Asians ratio, 0.52), and non-Hispanics (Hispanics-to-non-Hispanics ratio, 0.23).1
Unlike high-grade BP neuroendocrine tumors (large cell and small cell), typical and atypical BP carcinoid tumors rarely occur in combination with other adenocarcinomas; however, their risk of occurring synchronously with breast or prostate cancer is slightly elevated.5,6
Pathology
The WHO histologic classification of BP neuroendocrine tumors is based on specific morphologic features: organoid or trabecular growth pattern, palisading of the tumor cells around the periphery of tumor nests, and the formation of rosette structures. Although the WHO places these four subtypes under the general heading of BP neuroendocrine tumors, histopathologic and immunochemical studies support the idea that BP carcinoid tumors are distinct from those of the more malignant and higher-grade neuroendocrine tumors.7
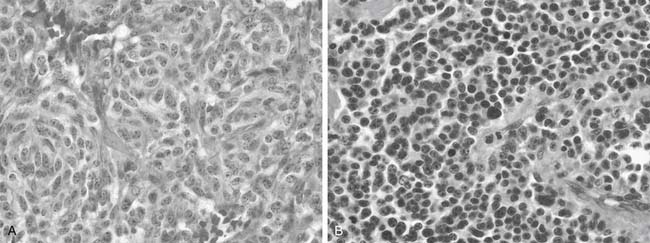
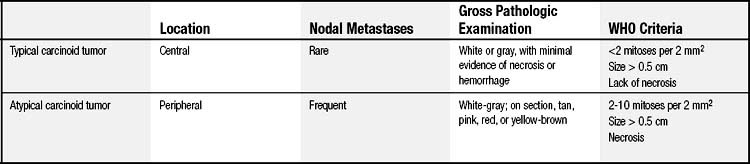
The majority of BP carcinoid tumors are typical and tend to be centrally located.8 Davila and coworkers reported that 75% of BP carcinoid tumors arise in the lobar bronchi, 10% in the main-stem bronchi, and 15% peripherally.9 Bronchoscopic evaluation of typical carcinoid tumors demonstrates sessile, red-brown to bluish tan endobronchial masses with variable vascularity and a smooth surface. On gross pathologic examination, typical carcinoid tumors appear as a white or gray cut surface with minimal evidence of hemorrhage or necrosis.10 The WHO diagnostic criteria for typical carcinoid tumors include a 0.5-cm or larger tumor with carcinoid morphology, with fewer than two mitoses per 2 mm2, and without necrosis (Fig. 22-1A and Table 22-1).11
In contrast, atypical carcinoid tumors tend to be located peripherally in the lungs.8 On gross pathologic examination, they appear white-gray on section but can be tan, pink to yellow brown, or red.12 The WHO diagnostic criteria for atypical carcinoid tumors include a tumor with carcinoid morphology with two to 10 mitoses per 2 mm2, with or without necrosis (see Fig. 22-1B and Table 22-1).11
Genetics and Biochemistry
Leotlela and colleagues report development of BP neuroendocrine tumors as a result of loss of heterozygosity in multiple chromosomes, including 3p, 11q13 (MEN1 gene), 13q13 (the retinoblastoma [RB] gene), and 17p13 (p53 gene).13 Loss of heterozygosity at chromosome 3p is the most frequent change in BP neuroendocrine tumors and has been observed in 40% of typical carcinoid tumors and 73% of atypical carcinoid tumors.14
BP carcinoid tumors are known to occur, although rarely, as components of familial endocrine cancer syndromes—namely, multiple endocrine neoplasia I (MEN-1). Inactivation of the MEN1 gene by mutation is seen in approximately 47% of typical carcinoid tumors and 70% of atypical carcinoid tumors.15–17 A study of 129 patients with MEN-1 found that six individuals (5%) had been diagnosed with BP carcinoid tumors on initial diagnosis.18 A review of the chest computed tomography (CT) scans of 32 of these patients showed that 12 patients had pulmonary nodules that were suspected to be BP carcinoid tumor, and four of these were histologically confirmed as BP carcinoid tumor. Therefore, it is recommended that patients with a diagnosis of MEN-1 be screened for BP carcinoid tumors with chest CT scanning every 3 years beginning at 20 years of age.18,19
The p53 gene is important for maintaining genomic stability in addition to numerous other functions. Loss of heterozygosity or abnormal expression of the p53 locus has been detected in 4% of typical carcinoid tumors and 29% of atypical carcinoid tumors.20,21 Kobayashi and coworkers evaluated the frequency of p53 protein expression in BP neuroendocrine tumors and found that typical carcinoid tumors demonstrated 0% expression, whereas atypical carcinoid tumors have 20% expression.22
E-cadherin and β-catenins, transmembrane glycoproteins involved in cell–cell adhesion, are expressed in BP carcinoid tumors at levels of 50% and 37%, respectively, which is a lower level than their expression in high-grade BP neuroendocrine tumors.23 A decrease in expression of E-cadherin and β-catenin also correlates with lymph node metastases, suggesting that an abnormal E-cadherin or β-catenin expression pattern is an independent predictor of atypical carcinoid tumor progression.24
Clinical Presentation
Because of the high proportion of centrally located BP carcinoid tumors,9 most patients are symptomatic at presentation. The most common symptoms are cough, hemoptysis, and pneumonia, representing the consequences of endoluminal obstruction secondary to the tumor. Symptoms may be present for many years before diagnosis, and, almost entirely, they reflect the anatomic location of the lesion as opposed to the secreted bioactive products.25 Interestingly, a population-based study in Denmark observed that 24% of typical carcinoid tumors and 7% of atypical carcinoid tumors were discovered on autopsy.25
Carcinoid syndrome (diarrhea, flushing, wheezing, and heart disease) as a presentation of BP carcinoid tumors is rare (1% to 3%) and is associated with liver metastases. the incidence of liver metastases is very rare (typical carcinoid tumor, 2%; atypical carcinoid tumor, 5%).26,27
Cushing syndrome (ectopic production and secretion of adrenocorticotropic hormone [ACTH]) can occur in 2% of patients with BP carcinoid tumors; however, less than 1% of those with Cushing syndrome have a BP carcinoid tumor.28 Aniszewski and colleagues studied 106 patients with ectopic production of ACTH and found that BP carcinoid tumors (25%) were the most frequent cause, followed by islet cell tumors (16%) and small cell lung cancer (11%).29
Other rare endocrinopathies that afflict patients with BP carcinoid tumors include acromegaly (increased production and secretion of growth hormone), hypercalcemia, and hypoglycemia.30,31
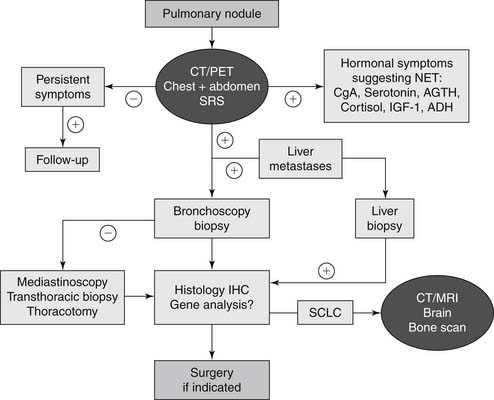
Diagnosis
Imaging
Chest radiographs are nonspecific for BP carcinoid tumors. Those tumors that do appear are often isolated, well-defined hilar or perihilar masses. Suspect lesions should be examined further with chest CT (Fig. 22-2). The usual CT appearance of a typical carcinoid tumor is that of a well-defined, spherical to ovoid mass that exerts a mass effect or obstruction of airways. They are often vascular and more often centrally located. In contrast, atypical carcinoid tumors are usually located peripherally and 30% have calcifications (Fig. 22-3).8
A majority of BP neuroendocrine tumors express (80%) somatostatin receptors, predominantly subtype 2.32 Somatostatin receptor scintigraphy uses radiolabeled somatostatin analogs (indium-111–labeled octreotide and 111In-lanreotide) to locate BP neuroendocrine tumors.33 This imaging modality is rarely used, however, in the diagnosis or workup of BP carcinoid tumors.
Positron emission tomography (PET) detects uptake of radiolabeled biological fluorodeoxyglucose labeled with radioactive fluoride (18F) (FDG) by neoplastic cells. FDG-PET has shown false-negative results with solitary BP carcinoid tumors, as they are usually hypometabolic on FDG-PET34; however, a more recent retrospective study by Marom and coworkers examined 192 T1 lung cancers, six of which were BP carcinoid tumors, and five of the six were detected using FDG-PET.35 Chong and colleagues reviewed seven FDG-PET scans of two typical carcinoid tumors and five atypical carcinoid tumors for the maximal standardized uptake value (SUV). The two typical carcinoid tumors had an SUV range of 3.2 to 3.4, with both specimens exhibiting less than mediastinal uptake. Three of the five atypical carcinoid tumors had higher than mediastinal uptake (4.0 to 7.1). One of the five atypical carcinoid tumors had a maximal SUV of 1.7, with an ipsilateral hilar lymph node measuring 11.2 SUV.36 Given the propensity for false-negatives in FDG-PET scans, a lesion that is suspected of being a carcinoid tumor on CT should be resected if the patient is operable.
Bronchoscopy
Rivera and coworkers reviewed nearly 3800 patients with central, endobronchial lesions and found the overall sensitivity of flexible fiberoptic bronchoscopy for detecting lesions was 88%.37 Because the majority (75%) of BP carcinoid tumors are centrally located, they are amenable to bronchoscopic evaluation. Even with direct visualization of BP carcinoid tumors, it is difficult to distinguish typical from atypical carcinoid tumor with the small biopsy typically obtained by flexible bronchoscopy. As 5% to 20% of typical carcinoid tumors and 30% to 70% of atypical carcinoid tumors metastasize, lymph nodes should be assessed to adequately stage the BP carcinoid tumor.12
In the past, a feared complication of flexible bronchoscopy was major hemorrhage; however, this is now rare. In a review of 587 biopsies by flexible and rigid bronchoscopy, significant hemorrhage occurred in 15 patients (2.6%), with only four (0.7%) requiring emergency intervention for massive uncontrollable hemorrhage.38 To reduce the risk of hemorrhage, an epinephrine solution can be administered through the bronchoscope before the biopsy. In the event of significant bleeding that is difficult to control, a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser is helpful.39 In summary, it is safe to bronchoscopically biopsy a suspected carcinoid tumor.
Tumor Markers
Serotonin and urinary 5-hydroxyindoleacetic acid are well-known markers of hormonally active carcinoid tumors; however, they are not specific for BP carcinoid tumors. Chromogranin A elevation in plasma is a relatively (75%) sensitive marker of BP carcinoid tumors.40 Care must be taken in patients with renal impairment or atrophic gastritis, and during proton-pump inhibitor therapy, as these conditions cause elevations in chromogranin A and thus false-positive results. In clinical practice, there is no evidence that measurement of tumor marker levels adds value or alters patient management, so they are not routinely performed.
Staging
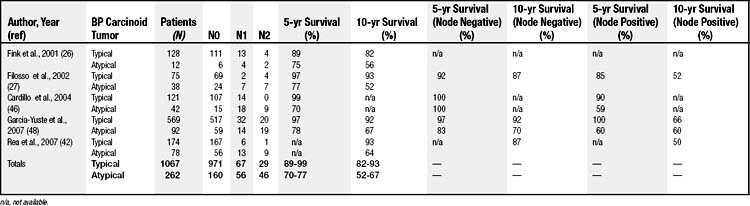
The TNM classification for lung cancer remains the most useful staging classification for BP carcinoid tumors. Fink and colleagues26 analyzed nearly 142 cases of BP carcinoid tumors (128 typical and 14 atypical) and found that 87% of typical carcinoid tumors were without lymph node metastases, 10% demonstrated N1 disease (ipsilateral hilar lymph node involvement), and 3% demonstrated N2 disease (ipsilateral mediastinal lymph node involvement). None were found to exhibit N3 disease. Two (1.5%) patients with typical carcinoid tumor presented with distant metastases. Of the atypical carcinoid tumors, 43% were N0, 29% demonstrated N1 disease, 14% demonstrated N2 disease, and 14% exhibited N3 disease. Three patients (14%) with atypical carcinoid tumor presented with distant metastases.26
Management and Treatment
Surgery
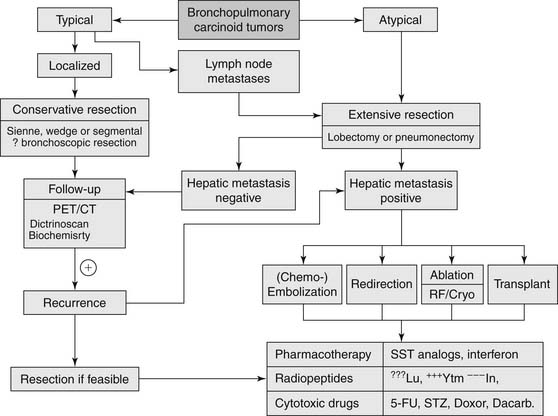
Complete surgical resection with preservation of normal lung tissue remains the only curative treatment of BP carcinoid tumors (Fig. 22-4).7 Centrally located typical carcinoid tumors should be resected using lung- and parenchyma-sparing resections such as a sleeve resection, or an anatomic segmentectomy.27,41 Surgical treatment of typical carcinoid tumors does not require a wide margin of resection, as local recurrence is rare.42 Ferguson and coworkers in a multicenter retrospective study found that a wide nonanatomic wedge resection or segmentectomy was justified in the case of peripheral typical carcinoid tumors because of the low likelihood of local recurrence.43
Although most agree that a nonanatomic resection, when possible, is acceptable in the management of typical carcinoid tumors, the surgical approach for atypical carcinoid tumors is typically a lobectomy or, rarely, a pneumonectomy. Because of the greater propensity of atypical carcinoid tumors to metastasize to lymph nodes, a more extensive and aggressive resection (lobectomy, bi-lobectomy, and pneumonectomy) and nodal dissection is recommended.9,44,45
Surgical resection of BP carcinoid tumors should be combined with ipsilateral mediastinal lymph node dissection or sampling.42 Lymph node dissection is justified by the possibility of lymph node metastasis, which has an incidence of 4% to 13% in typical carcinoid tumors and 28% to 67% in atypical carcinoid tumors.26,27,42,46
Endobronchial Management
Bertoletti and colleagues recently conducted a study of 18 patients (all with typical carcinoid tumor, strict endoluminal disease, and no evidence of lymph node invasion) treated with flexible bronchoscopy and cryotherapy. Patients were followed for 55 months, and there was one recurrence in 7 years, with no long-term complications.47 Although these results are encouraging, endobronchial resection should be reserved for patients who are not amenable to surgical intervention.
Prognosis
With an increase in the number of chest imaging studies as well as the enhanced quality of the imaging techniques, there has been an increase in the reported incidence of BP carcinoid tumors. Despite what could be perceived as earlier detection of all carcinoid tumors, a review of the SEER database demonstrates that there is a decrease in the 5-year survival rate of all patients with carcinoid tumors (including atypical ones) over the past 30 years.2 Reasons for this observation are unclear; it may be related to an increase in the incidence of atypical carcinoid tumor histology.
Patients with typical carcinoid tumors have better prognoses than those with atypical carcinoid tumors. This difference is primarily explained by the differences in tumor biology between the two (Table 22-2). Recent studies have shown that the 5- and 10-year survival rates for patients with typical carcinoid tumors are 89% to 99% and 82% to 93%, respectively, whereas the 5- and 10-year survival rates for patients with atypical carcinoid tumors are 70% to 77% and 52% to 64%, respectively.26,27,42,46
The prognostic significance of lymph node involvement has been addressed by many studies in the past decade (see Table 22-2). Cardillo and coworkers and Garcia-Yuste and colleagues have demonstrated a significant 5-year survival difference for patients with node-positive atypical carcinoid tumors disease (59% and 60%, respectively) when compared with node-negative disease (100% and 83%, respectively). For typical carcinoid tumors, the presence or absence of nodal disease does not correlate with survival.46,48 Both Filosso and associates and Rea and coworkers have found there to be significantly better 10-year survival in patients diagnosed with BP carcinoid tumors and N0 disease regardless of histology (87% and 87%, respectively) when compared with those with N1 and N2 disease (52% and 50%, respectively).26,42
CARCINOID TUMORLETS
Gustafsson and colleagues discussed diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH), a rare preneoplastic condition involving proliferation of pulmonary neuroendocrine cells and neuroepithelial bodies. When this proliferation extends beyond the basement membrane, the group of cells is identified as a tumorlet. A BP tumorlet is a nodular proliferation of neuroendocrine cells that constitutes a nodule less than 5 mm, and those that proliferate to greater than 5 mm are classified as BP carcinoid tumors.7 DIPNECH can be both an adaptive response, as seen in persons living at high altitudes, and a reactive response to lung injury, as seen in patients with obliterative bronchiolitis,49 chronic cough,50 and interstitial lung disease.51
Carcinoid tumorlets and carcinoid tumors have long been associated, but a definitive relationship has not been established. Studies have demonstrated the presence of tumorlets in normal lungs or in association with carcinoid tumors.52,53 There have also been case reports of isolated peribronchial54 and hilar55 lymph node metastases that point to the possible neoplastic potential7 of carcinoid tumorlets. In each of these circumstances, the lymph nodes appeared grossly normal but were found to have microscopic metastases on pathologic examination.
Pathology and Diagnosis
Carcinoid tumorlets develop from Kulchitsky cells, which are hyperplastic neuroendocrine cells in the bronchial and bronchiolar mucosa. It has been suggested that these cells secrete neuropeptides that can elicit peribronchiolar reaction leading to fibrotic lung disease.49,56
The majority of pulmonary carcinoid tumorlets are often asymptomatic, even if multiple tumorlets are present. Pulmonary symptoms, dysfunction, and radiologic abnormalities have been attributed to multiple tumorlets.57
Aubry and coworkers57 retrospectively reviewed 28 patients (26 were women) and their imaging. Patients were grouped according to results of imaging studies: multiple nodules (17), solitary nodule/opacity (seven), and obstructive lung disease (four). Those with multiple nodules were most likely to present with cough (59%) and dyspnea (47%); however, 41% of the patients had no presenting symptoms. Nearly one half of the patients (47%) with multiple nodules were nonsmokers, but 59% had test findings of obstructive pulmonary function. Thirteen patients with multiple nodules had both a carcinoid tumor and tumorlet, and four had only tumorlets. Three patients (11%) with a dominant carcinoid tumor had lymph node metastases.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree