Fig. 6.1
The flow volume loop in fixed central airway obstruction reveals blunting of both the inspiratory and expiratory portions of the loop
Conventional chest X-rays are neither sensitive nor specific for the diagnosis of ISS. Chest CT allows a more precise assessment of the tracheal anatomy and also provides information on the mediastinum, which by definition should appear normal in ISS (i.e. no extrinsic compression). High-resolution CT using multi-row detectors now allows for three-dimensional reconstruction and virtual bronchoscopy images that can help define the type of stenosis (concentric, complex, hourglass) before invasive techniques. In addition, dynamic CT with expiratory views allows identification of dynamic collapse due to tracheomalacia occasionally associated with ISS [1, 2].
Bronchoscopy is the gold standard for the diagnosis of tracheal stenosis (Fig. 6.2). Typically, flexible bronchoscopy is used first in order to determine the location, extent and complexity of the stenosis. EBUS (endobronchial ultrasonography) can document the thickening of lamina propria of tracheal mucosa without cartilage involvement. This diagnostic tool can be helpful in both differentiating ISS from other diseases that usually involve cartilage (i.e., chondromalacia, relapsing polychondritis) and in assessing the extent and complexity of the tracheal stenosis. Occasionally, the stenosis is severe enough that only pediatric or ultrathin bronchoscopes may be used. In this situation, however, extreme caution is needed as edema or inflammation caused by the endoscopic procedure can result in life-threatening airway obstruction. In these cases, proceeding directly to rigid bronchoscopy may allow better management of the airway in a controlled operating room setting. High-frequency ventilation can be used with rigid bronchoscopy and is very helpful in this setting. When obtained, biopsies, by definition, show no evidence of granulomatous inflammation or malignancy. The typical histological finding consists of cheloidal fibrosis with dilatation of mucous glands and normal cartilage [12]. As mentioned above, studies evaluating the presence of estrogen and progesterone receptors have not been conclusive.
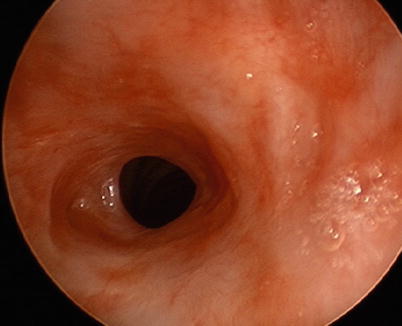

Fig. 6.2
Bronchoscopic view of idiopathic subglottic stenosis in a 38-year-old woman. The tracheal lumen is narrowed to 4 mm diameter
The definitive treatment of ISS consists of single-stage laryngotracheal resection with or without posterior membranous tracheal wall flap. The largest series published by Ashiku et al. included 73 patients, 67 of whom had excellent long-term results without the need for further endoscopic or surgical interventions with a mean follow-up of 7.9 years [17]. In contrast, the largest case series on endoscopic management of ISS reported recurrence requiring re-intervention in 30 % of patients at 6 months and 87 % at 5 years [18]. While the results would argue for surgical intervention in the majority of patients, it cannot be overemphasized that patients should be referred to centers of excellence with expertise in the management of this exceedingly rare disease. Endoscopic treatment varies and is based on expert opinion. It may include simple dilatation using rigid bronchoscopy with barrels of increasing diameter, which may be preceded by radial cuts using laser. Others have used flexible bronchoscopy with balloon dilatation. We tend to favor rigid bronchoscopy which offers the safety of a more secured airway. Local applications of mitomycin C and/or intralesional injections of corticosteroids are sometimes used to prevent recurrences, though the evidence for this practice is scarce. In fact, some reports suggest that the excessive use of mitomycin C may be associated with subsequent tracheal stenosis from excessive fibroproliferation [19]. Airway stents are occasionally considered, but their use may be associated with recurrent tracheal trauma that could jeopardize future definitive surgical treatment. Tracheostomy is thought to present the same risks and is usually discouraged. The value of empiric medical therapy including inhaled steroids and empiric proton-pump inhibitor remains unclear at this time, though documented gastroesophageal reflux disease should be aggressively treated.
Idiopathic Subglottic Stenosis: Key Points
Female predominance
Location: subglottis
Histology: cheloidal fibrosis of the lumina propria with preservation of the tracheal cartilage.
By definition a diagnosis of exclusion, secondary causes of tracheal stenosis should be excluded
Tracheobronchopathia Osteochondroplastica
Clinical Vignette
A 55-year-old man is referred to urology for prostatectomy after being diagnosed with prostate adenocarcinoma. He is a never smoker but was diagnosed with chronic obstructive pulmonary disease a few years before and uses a beta-2-agonist inhaler on an as-needed basis as well as inhaled steroids. He has moderate obstruction on his pulmonary function test but his diffusing capacity is normal. He is considered low risk for surgery from a respiratory standpoint and undergoes an uneventful radical prostatectomy. After the procedure, the anesthesiologist recommends a pulmonary consultation because of difficulties during endotracheal intubation before the procedure, requiring placement of a smaller-diameter endotracheal tube. A chest CT scan shows prominent calcified tracheal nodules sparing the posterior membrane with normal lung parenchyma. Fiberoptic bronchoscopy confirms the diagnosis of tracheobronchopathia osteochondroplastica.
With less than 400 cases reported in the literature, tracheobronchopathia osteochondroplastica (TPO) is one of the most rarely encountered tracheal diseases [20]. It is characterized by the non-malignant growth of bone and/or cartilaginous submucosal nodules that protrude into the lumen of the trachea and proximal main stem bronchi. As they arise from the tracheal cartilages, these nodules typically spare the posterior membrane, which generally helps distinguishing this diagnosis from other tracheal diseases, especially amyloidosis. This entity is likely underreported as affected patients are generally asymptomatic or have mild respiratory symptoms.
TPO affects both males and females with equal frequency, and does not appear to be influenced by smoking. Most patients are middle-aged adults though few cases have been reported in children. The pathogenesis of the disease remains obscure, although some have suggested that ongoing irritation from chronic cough may eventually lead to metaplasia of the elastic connective tissue. Biopsies of the lesions of TPO have revealed the presence of bone morphogenetic protein 2 and transforming growth factor beta-1, cytokines involved in extracellular matrix and bone formation [21]. An association with amyloidosis has been described, and some have suggested that TPO could be a manifestation of tracheobronchial amyloidosis, though the evidence supporting this assertion is limited to a few case reports. More likely, these two entities represent distinct tracheal diseases with overlapping clinical manifestations. Finally, Klebsiella ozonae, a bacteria responsible for the development of atrophic rhinitis, has been suggested as a possible cause for TPO as its presence was demonstrated in 20 % of TPO patients in a large case series [20, 22, 23].
In the majority of cases the presence of TPO is identified incidentally on the basis of chest CT demonstrating calcified submucosal nodular thickening, or during bronchoscopy. Occasionally, the confluence of osseous and cartilaginous nodules can lead to mass-like formation resulting in luminal narrowing and symptomatic tracheal stenosis. Laryngeal involvement may occasionally be seen as well. Hemoptysis, due to ulceration of the mucosa overlying these nodules, is a rare manifestation of the disease and generally minimal and self-limited. Cough, wheezing or stridor, hoarseness, recurrent infections (due to poor mucociliary clearance and post-obstructive infections) can occur as well. A characteristic presentation, as described in the case above, is that of difficult endotracheal intubation eventually leading to the diagnosis.
Pulmonary function studies are frequently normal, but occasionally may demonstrate an obstructive defect when the degree of tracheal narrowing causes significant airflow limitation. The central airway location of the disease can be identified by flow-volume loop showing plateau of the inspiratory or expiratory portion of the curve depending on whether the level of obstruction is extra-versus intrathoracic, respectively. In some cases (extensive disease or fixed obstruction), both the inspiratory and the expiratory portions may be abnormal [22].
Chest X-rays are rarely sensitive enough to suggest the diagnosis, but may occasionally show narrowing and irregularity the of the tracheal air column with calcified deposits. Chest CT reveals the characteristic calcified nodules arising from the anterior and lateral walls of the trachea with varying degrees of narrowing and irregular lumen (Fig. 6.3) [22–24]. As mentioned earlier, the posterior membrane is typically spared and, if involved, should suggest the possibility of alternative diagnoses, specifically amyloidosis and relapsing polychondritis, which can both result in significant central airway calcifications.
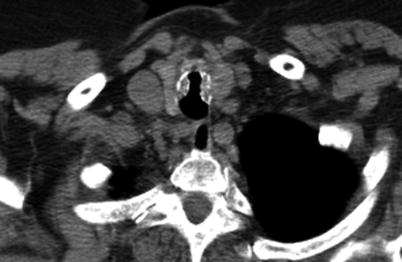

Fig. 6.3
CT scan of the chest of an 89-year-old woman with tracheobronchopathia osteochondroplastica. Partially calcified submucosal nodules are present in the tracheal walls with sparing of the posterior membranous wall
Bronchoscopy shows very characteristic lesions which can establish the diagnosis in most cases. Submucosal nodules protruding into the airway can be seen at all levels of the trachea (Fig. 6.4) but result in clinically significant narrowing (>50 %) in only a minority of patients, a threshold classically described as clinically significant based on data on tracheobronchomalacia (see below). While the posterior membrane is generally spared, progression of the nodule formation may eventually extend posteriorly in 15 % of patients. Biopsies are not mandatory to establish the diagnosis when palpation with forceps confirms the firmness of the calcified and/or osseous nodules. When biopsies are obtained, which can be difficult, they reveal the presence of submucosal cartilage and bone formation with occasional intraosseous bone marrow formation. Proximal main stem bronchi may be involved as well, but more distal airways are involved in less than 20 % of patients [22, 25].
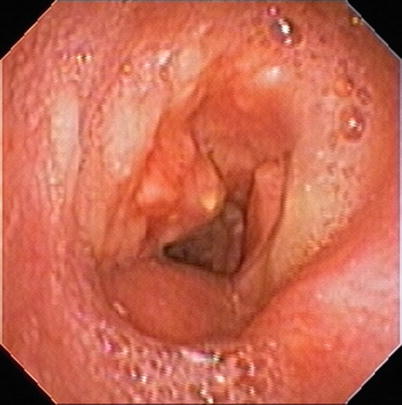

Fig. 6.4
Bronchoscopic view of tracheobronchopathia osteochondroplastica revealing osseous submucosal nodules projecting into the tracheal lumen
Treatment of TPO is difficult, and mainly supportive. Bronchopulmonary hygiene measures aimed at improving secretion clearance are of paramount importance in patients with recurrent infections due to impaired mucociliary function and post-obstructive infections. Immunizations should be updated. The definitive treatment of TPO is difficult, as the firm calcified and osseous nodules do not lend themselves well to endoscopic resection. Furthermore, the diffuse extent of the lesions along the tracheal walls often precludes any consideration of reconstructive surgery. When indicated, rigid bronchoscopy with resection of the nodules using gentle and careful pressure with the bevel of the bronchoscope is usually the most efficient, but tracheal injury may result. Other techniques have been described including laser-assisted mechanical debulking. An important caveat is that any consideration of endoscopic treatment should be symptom-driven, as the lesions of TPO are minimally progressive in most patients and follow a benign course [22, 25, 26].
TPO: Key Points
No gender predilection
Tracheal involvement typically spares the posterior membrane
Biopsies are not needed in typical cases
Differential diagnosis on CT imaging includes amyloidosis and relapsing polychondritis.
Tracheomalacia
Clinical Vignette
A 55 year-old man with known chronic obstructive pulmonary disease (COPD) is admitted to the pulmonary ward for his third episode of pneumonia this year. His cough has worsened and productive of purulent sputum along with increased shortness of breath. The chest X-ray reveals consolidation in the right lower lobe. Pulmonary function studies reveal severe obstruction, markedly worse than noted 2 years prior to admission during an outpatient evaluation. A chest CT scan confirms the right lower lobe infiltrate but is otherwise unremarkable. A bronchoscopy is undertaken to explore the possibility of endobronchial lesion. The bronchoscopy reveals severe tracheobronchomalacia from excessive dynamic airway collapse secondary to severe laxity of the posterior membrane. The pulmonary service is consulted for management recommendations.
Tracheomalacia (from the Greek malakia, i.e., softness) refers to a weakness of the trachea that results in increased compliance and excessive reduction in the tracheal luminal dimensions during normal or forced expiration and/or inspiration. Because the trachea is mainly intrathoracic (lower two-thirds approximately), most of the changes noted occur during expiration, as the airway tethered to the surrounding thoracic structures remains relatively normal during inspiration [27, 28]. The extrathoracic portion of the trachea is occasionally involved as well and inspiratory collapse with audible stridor may then occur. When the proximal bronchi are involved, the appropriate terminology is tracheobronchomalacia. The distinction is essentially semantic as the manifestations and clinical implications are identical.
Tracheomalacia may be diffuse, as seen in excessive dynamic airway collapse, or focal, as seen in complications of tracheostomy for example. In general, focal lesions are more easily amenable to endoscopic or surgical treatment, emphasizing the importance of a careful endoscopic examination. It has been argued that tracheomalacia should only refer to excessive tracheal weakness from structural insufficiency of the tracheal cartilaginous rings and should be distinguished from excessive dynamic airway collapse, related to excessive laxity of the posterior membrane. As these conditions result in the same manifestations and are managed in a similar fashion, this distinction is not particularly helpful.
The vast majority of cases described in children are congenital and include mucopolysaccharidoses (such as Hurler syndrome and Hunter syndrome) and Williams-Campbell syndrome (absence of cartilages resulting in loss of structural support). Other causes of tracheomalacia in children include compression of the trachea by vascular rings or right-sided aortic arch. The persistent compression of the trachea is thought to result in chronic ischemic changes and cartilage destruction eventually leading to focal tracheomalacia. Bronchiectasis is likely to develop over time as a consequence of recurrent lung infections from retained secretions and, as such, tracheomalacia should be considered in the differential diagnosis of diffuse bronchiectasis. Various types of tracheomalacia are described in adults. As for children, prolonged tracheal compression from surrounding structures may eventually result in focal tracheomalacia. This includes chronic endotracheal intubation with excessive cuff pressure, tracheostomy or other forms or trauma to the airway, extrinsic compression from tumoral processes or lymph nodes and thyroid goiters. Other causes include infections (such as tuberculosis) or, rarely, heart-lung transplant (as the anastomosis is located in the lower trachea). Some inflammatory conditions may result in diffuse tracheomalacia, such as relapsing polychondritis (discussed separately) and inhalational injuries (including recurrent aspirations). Tracheomalacia from excessive dynamic airway collapse is typically observed in COPD, though occasionally will occur in never-smokers. Idiopathic tracheomalacia is relatively rare. One type of idiopathic tracheomalacia is Mounier-Kuhn syndrome, or tracheobronchomegaly, which typically manifests in adult life (also discussed separately) [27–34].
There are few descriptions of the histopathological changes associated with tracheomalacia. Autopsy studies have revealed atrophy of the longitudinal muscle fibers with or without cartilaginous destruction or absence of the cartilaginous support structure [27, 28]. Inflammatory cellular infiltrates may also be noted in some instances, such as in relapsing polychondritis [35].
Clinical manifestations vary based on the degree of luminal narrowing. Some asymptomatic patients may decompensate only during episodes of respiratory infections or during sleep (due to sleep-related respiratory changes and recumbent position). Symptomatic patients may experience wheezing, typically described as monophonic, and, rarely, stridor when the extrathoracic portion of the trachea is involved. Recurrent infections are secondary to impaired mucous clearance and are a common presentation. They may eventually lead to the development of bronchiectasis, aggravating the obstructive syndrome and predisposing patients to yet further infections. Cough may be severe and occasionally result in cough-induced syncope. These clinical manifestations are non-specific, however, and often result in delayed diagnosis with patients incorrectly diagnosed to have chronic bronchitis or refractory asthma [27, 28].
Pulmonary function studies are typically consistent with obstruction. The severity of the obstructive syndrome is directly proportional to the degree of tracheomalacia. Obstruction that is considered out-of-proportion to the smoking history in a COPD patient should suggest tracheomalacia from excessive dynamic airway collapse. One clue to the diagnosis is the presence of a plateau on the expiratory portion of the flow-volume curve following a reduced peak expiratory flow rate. Oscillations of flow, similar to those noted in obstructive sleep apnea patient have been reported as well. If the extrathoracic portion of the trachea is involved, a plateau may also be noted on the inspiratory curve [27, 28, 36].
Chest X-rays are usually inadequate in the diagnosis of tracheomalacia, though they may occasionally suggest a mediastinal process that may be responsible for focal extrinsic compression and lead to additional studies. Computed tomography (CT) images may also be misleading if obtained only during inspiration, as the tracheal dimensions are generally normal under these conditions (unless the extrathoracic trachea is involved as well). If the diagnosis of tracheomalacia is suspected, dynamic CT study should be obtained by requesting expiratory images. The diagnostic accuracy of dynamic CT approaches that of bronchoscopy and allows precise measurements of the luminal diameter changes and extent of tracheomalacia [1, 2]. Multi-row detector spiral CT allow for image acquisition within seconds and is generally obtainable even in the most dyspneic patients. The type of luminal narrowing can be accurately characterized by CT. Reduction in the anteroposterior diameter is described as crescent-shaped (“frown sign” on CT images) (Fig. 6.5), while reduction in the sagittal diameter has been referred to as “saber-sheath trachea”. This latter presentation is more common in patients with emphysema and is thought to result from chronic cough with microfractures of the cartilages and lateral compression from hyperinflated upper lobes.
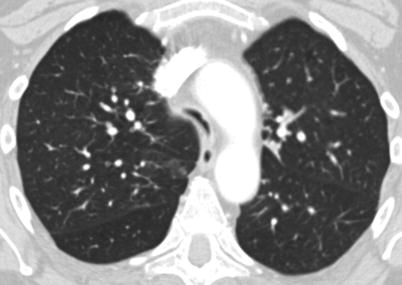

Fig. 6.5
CT scan of the chest of a 57-year-old man with severe tracheomalacia demonstrating the “frown sign”
The criteria for tracheomalacia on CT are identical to those used during bronchoscopy. By convention, airway collapse is considered significant if the minimum luminal diameter is 50 % or less than the maximum diameter. Luminal narrowing down to 25 % is considered moderate, and complete collapse is designated as severe [27]. These criteria are supportive of the diagnosis but should be considered diagnostic only in the appropriate clinical setting, as several studies have shown that a majority of healthy controls can experience narrowing >50 % during forced expiratory maneuvers [37, 38]. For this reason, a 75 % narrowing cutoff has been proposed by some for diagnosing tracheomalacia.
Bronchoscopy remains the diagnostic gold standard, although it does not provide the same quantitative measurements of airway diameter assessed by CT imaging. Again, a narrowing >50 % is considered consistent with the diagnosis, but is based on a semi-quantitative assessment by the bronchoscopists. Bronchoscopy should be performed with conscious sedation as it allows maneuvers of cough and forced expiration not possible under general anesthesia. Morphometric bronchoscopy has been proposed as potential tool to allow quantitative analysis of airway dimensions via software analysis of digital bronchoscopic images, but its use remains experimental at the present time. One major advantage of bronchoscopy over CT is the possibility to identify endoluminal pathology responsible for the tracheal narrowing which may be missed by CT. In addition, bronchoscopic interventions may be possible in the same setting or allow adequate planning for further interventions.
Treatment of tracheomalacia should be individualized according to the type, the extent and the etiology of the tracheomalacia. Treatment of the underlying cause, when possible, is warranted (such as systemic anti-inflammatory treatment of relapsing polychondritis or resection of a mediastinal mass). If possible, tracheomalacia in children should be observed as it may resolve spontaneously as the patients get older and the cartilaginous support structures mature. Non-invasive measures such as positive-pressure ventilation during sleep have been suggested, particularly in the context of excessive dynamic airway collapse, and may allow improved airflow, though the supportive evidence overall remains scarce [27, 28, 39]. Focal lesions are sometimes amenable to tracheal resection and end-to-end anastomosis, which is the considered the definitive treatment. When the tracheomalacia is diffuse, or when the patient is not deemed an appropriate candidate for surgical treatment, endoscopic interventions may be helpful. Rigid bronchoscopy with silicone stent placement may result in significant improvement in lung function and symptoms. Migration of the choke point beyond the extremities of the stent may limit its efficacy however, and excessive stent length can lead to further impairment of mucous clearance and predispose patients to recurrent infections. Inhalation of nebulized saline is warranted after stent placement to avoid inspissation of mucous and occlusion of the stent. Metallic stents, while similarly efficacious in reestablishing airway patency, should be avoided in benign airway diseases, as they are associated with serious long-term complications. As opposed to silicone stents, they can be difficult to remove when left in place for prolonged periods of time.
An alternative option for diffuse diseases is surgical tracheobronchoplasty, which consists of reinforcing the posterior membrane of the central airways with prosthetic material such as Marlex mesh, effectively resulting in splinting of the airway [40, 41]. Potential candidates for this procedure should be selected on the basis of a favorable response to silicone stenting [27]. Long-term stenting is an option for those who do respond but are not considered acceptable candidates for this invasive procedure. A recent study showed that airway stabilization via tracheoplasty or stenting for COPD-associated excessive dynamic airway collapse results in significant improvement in quality of life and physiologic parameters.
Finally, tracheostomy may occasionally be performed if the area of narrowing can be successfully bypassed.
Tracheomalacia: Key Points
Increased compliance of the trachea with collapsibility
May be idiopathic or secondary
May be focal or diffuse
Effects of endotracheal stent placement can predict response to surgical management
Tracheobronchomegaly
Clinical Vignette
A 30-year-old man with a history of recurrent infections of the lower respiratory tract and refractory asthma presents to the emergency department for the sudden onset of shortness of breath. A chest X-ray reveals a right-sided pneumothorax and chest tube thoracostomy is performed for management. A chest CT is obtained to assess for underlying parenchymal lung disease, which reveals significant bronchiectasis that predominate in the lower lobes with marked enlargement of the central airways. A bronchoscopy later confirms the diagnosis of tracheobronchomegaly. Several tracheal diverticuli are noted during the bronchoscopic examination.
Idiopathic tracheobronchomegaly, also called Mounier-Kuhn syndrome, was first reported in an adult patient in 1932. Since, over 100 cases have been reported in the literature [27]. It is considered a congenital disease affecting the trachea and proximal bronchi resulting in abnormal enlargement of the airway, leading to tracheobronchomalacia with impaired secretion clearance and recurrent infections. Though occasionally identified during childhood, the disease more often presents later in life after development of bronchiectasis and recurrent infections prompt further investigations.
Abnormal enlargement of the central airways has been described in association with a variety of conditions including connective tissue diseases such as Marfan syndrome, Ehlers-Danlos syndrome and ankylosing spondylitis. Congenital diseases have also been reported in association with tracheobronchomegaly and include Bruton’s agammaglobulinemia, Kenny-Caffey syndrome, ataxia telangiectasia and Brachmann-de Lange syndrome. Finally, similar to traction bronchiectasis, fibrotic infiltrative lung processes have occasionally been reported to cause enlargement of the central airways tethered to the surrounding fibrotic lung parenchyma. These conditions include idiopathic pulmonary fibrosis and other chronic parenchymal lung diseases such as sarcoidosis, rheumatoid-associated interstitial lung disease, chronic histoplasmosis and idiopathic pleuroparenchymal fibroelastosis. The term Mounier-Kuhn syndrome should be reserved to the idiopathic form of the disease and is also called idiopathic giant trachea. Several familial cases have been described [42–48].
Mounier-Kuhn syndrome tends to affect males with a higher frequency [46, 48]. Although the anatomical anomalies are generally present in childhood, the symptoms usually become evident in adulthood, in the 30s or 40s. A significant percentage of patients with Mounier-Kuhn syndrome are asymptomatic and diagnosed on the basis of abnormalities identified on imaging studies (typically chest CT) obtained for other reasons. Associated symptoms mainly consist of chronic cough and shortness of breath, recurrent infections, increased sputum production and bronchiectasis. Occasionally, patients may report episodes of hemoptysis. Rare cases of pneumothorax have been reported [49].
The pathophysiology of the Mounier-Kuhn syndrome remains to be elucidated. Histopathology data are limited, but suggest that the tracheal and bronchial walls contain abnormal connective tissue responsible for weakness of the central airways leading to significant tracheobronchomalacia. Atrophy of the smooth muscles and elastic component of the airway walls has been described in autopsy studies [50–52]. The resultant tracheobronchomalacia causes reduction of airflow, impaired secretion clearance and recurrent infections ultimately leading to bronchiectasis. Outpouchings of the tracheal mucosa, or airway diverticuli, may develop over time, and are highly suggestive of the diagnosis when identified by chest CT imaging. These may result in additional secretion retention potentially increasing further the risk of infectious complications. The prognosis of the disease varies widely, but severe cases can progress to respiratory failure.
Pulmonary function studies are typically consistent with an obstructive syndrome. As described in other types of tracheomalacia, an expiratory plateau may be identified suggesting central airway obstruction. Restrictive defects are rare but may occasionally be seen when pulmonary fibrosis is present.
Chest X-ray may occasionally suggest the diagnosis which is confirmed by the presence of central airway enlargement on chest CT (Fig. 6.6). The diagnosis is established when the airway diameter exceeds the following cutoffs, which represent 3 standard deviations above the norm, on average 2.4 cm for the right main stem bronchus, 2.3 cm for the left main stem bronchus and 3 cm for the trachea. [1, 3] The upper limits of normal for coronal and sagittal diameters in men are 25 and 27 mm respectively, and 21 ad 23 mm in women, respectively [3].
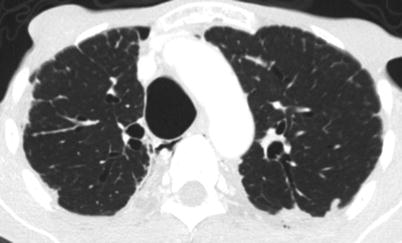

Fig. 6.6
CT scan of the chest of a 66-year-old man with tracheobronchomegaly. The antero-posterior diameter is 37 mm
Treatment of Mounier-Kuhn syndrome is challenging. The size of the central airways often precludes endoscopic stenting due to the lack of appropriately sized stents. The largest stent deployed in a case of tracheomegaly in association with Marfan syndrome had an outer diameter of 2.8 cm and had to be custom-made [53]. Despite this, a recent case series reports improvements in quality-of-life indices and physiologic parameters after both endoscopic stenting and surgical tracheobronchoplasty in patients with Mounier-Kuhn syndrome [54]. One anecdotal report described lasting improvement with low-power yttrium aluminum perovskyte laser treatment of the posterior membrane of a Mounier-Kuhn patient causing effective retraction of the tissues, though the safety of this approach remains in question [55]. Supportive interventions such as non-invasive positive-pressure ventilation at night, bronchopulmonary hygiene measures and appropriate and timely antibiotic treatment and immunizations are recommended. Few patients with Mounier-Kuhn syndrome have undergone lung transplantation and, as for cases of severe bronchiectasis, bilateral lung transplant is preferred over single-lung transplant.
Tracheobronchomegaly: Key Points
Male predominance
Diagnosis typically made in early adulthood
Recurrent infections and bronchiectases common
Endoscopic treatment is difficult due to the large size of the affected airways
Tracheopathies Associated with Systemic Diseases
The trachea and proximal main bronchi are sometimes involved in a variety of systemic diseases occasionally discovered during the work-up of the respiratory symptoms. Consideration of these diseases in patients with central airway disorders is warranted as they may influence management and prognosis. While a comprehensive review of all clinical entities potentially associated with central airway involvement is clearly beyond the scope of this chapter, we will review herein the most common offenders: relapsing polychondritis, granulomatosis with polyangiitis (formerly Wegener’s granulomatosis), sarcoidosis and amyloidosis.
Relapsing Polychondritis
Clinical Vignette
A 42-year old woman presents with shortness of breath and stridor. She has a past medical history significant for hearing loss of unclear etiology and mitral regurgitation. The physical examination reveals a saddle nose deformity and central wheezing on lung auscultation. A chest radiography reveals no apparent abnormalities. Blood work reveals increased inflammatory markers with elevated sedimentation rate and C-reactive protein. A Chest CT suggests tracheal narrowing and a bronchoscopy is performed. Endoscopic examination reveals subglottic stenosis, marked inflammation throughout the tracheobronchial tree and severe tracheobronchomalacia. Upon further questioning, the patient reports recurrent episodes of ear inflammation and a diagnosis of relapsing polychondritis is established.
Relapsing polychondritis is a rare type of autoimmune connective tissue disease that affects both males and females with equal frequency. It is characterized by recurrent episodes of inflammation involving various cartilaginous structures including ears, nose, upper airway (including the larynx), joints and cardiac valves (mitral and/or aortic valve regurgitation). In addition, the disease may also result in life-threatening complications affecting the kidneys and central nervous system (CNS). It is most commonly diagnosed in middle-aged adults [35, 56, 57].
Unilateral or bilateral ear inflammation is the most common presenting symptom and ultimately occurs in the vast majority of patients during the course of the disease. Approximately 30 % of patients will report hearing loss or dizziness related to vestibular involvement. This constellation of symptoms in patients with central airway involvement should suggest the diagnosis of relapsing polychondritis. The characteristic auricular chondritis seen in the majority of patients with relapsing polychondritis is not a feature of granulomatosis with polyangiitis. However differentiating relapsing polychondritis from granulomatosis with polyangiitis can be difficult because both diseases can manifest saddle nose deformity and tracheobronchial involvement; the possible overlap between these two entities has been discussed earlier. Biopsy of the tracheal cartilage shows degeneration with fibrous changes and inflammatory cell infiltration. The histologic picture is not absolutely characteristic and specific diagnostic tests are lacking.
Central airway involvement is common in patients with relapsing polychondritis. The largest case series reported by Ernst and colleagues included 145 patients, 31 of whom had evidence of airway involvement (21 %) with a majority being female (70 %). The respiratory manifestations consisted of subglottic stenosis in 8 patients (26 %), focal or diffuse tracheobronchomalacia in 15 patients (48 %) and focal stenosis in the remainder [58]. Other reports suggest that central airway manifestation may occur over time in approximately half of patients with relapsing polychondritis. Clinical manifestations are non-specific and include chronic cough, wheezing and/or stridor, and hoarseness in case of laryngeal involvement [56, 59].
Laboratory abnormalities are also generally non-specific and the diagnosis remains essentially clinical. Anemia of chronic disease may be present and eosinophilia is noted in approximately 10 % of patients. Inflammatory markers are elevated during periods of active disease but may be normal between exacerbations. They are helpful for monitoring the disease and for treatment decisions, but do not exclude the diagnosis when normal. Autoantibodies are sometimes present, consisting of antinuclear antibodies in approximately half of the patients. Rheumatoid factor and antiphospholipid antibodies are occasionally noted. Antineutrophil cytoplasmic antibodies (ANCAs) have also been described in relapsing polychondritis. Since patients with active limited granulomatosis with polyangiitis have a 40 % chance to be ANCA-negative, this laboratory test does not always allow clear distinction between relapsing polychondritis and granulomatosis with polyangiitis. There is strong support for an autoimmune process directed at some extracellular components of cartilage, but no particular antibody has been identified as either sensitive or specific for the disease. Anti-type II collagen antibodies, in particular, are found in a variety of other conditions and are thought to result from a non-specific immune reaction to cartilage destruction, rather than being true pathogenic antibodies. The utility of identifying these antibodies in clinical practice is unclear [60, 61].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


