Fig. 24.1
(a, b) Histopathological pattern of organizing pneumonia at surgical lung biopsy: buds of granulation tissue containing myofibroblasts and inflammatory cells embedded in a loose connective matrix, and filling the alveolar spaces without disruption of the parenchymal architecture. Mild inflammatory infiltrates of the alveolar interstitium
Intraalveolar fibrosis resulting from organization of inflammatory exsudates in OP is characterized by dramatic reversibility with corticosteroids, in sharp contrast with fibrosis in the other fibrosing idiopathic interstitial pneumonias, especially usual interstitial pneumonia (UIP), which is irreversible. The mechanisms governing the disappearance of myofibroblasts and fibroblasts from alveolar spaces in OP (spontaneously or with corticosteroids) are poorly understood. Apoptosis may play a role, as apoptotic activity is increased in the newly formed connective tissue in OP [15]. Intraalveolar buds in OP are also characterized by prominent capillarization resembling granulation tissue in cutaneous wound healing [16]. Vascular endothelial growth factor and basic fibroblast growth factor are widely expressed in intraalveolar buds, and angiogenesis mediated by these growth factors could contribute to the reversal of buds in OP [17].
Clinical Vignette
A 77-year old woman presented because of progressive dyspnea stage NYHA II, cough, fatigue, night sweats, anorexia, and loss of 10 kg over 1 year. A chest X-ray showed a left basal infiltrate. A course of antibiotic therapy had no effect, and the patient was referred to a respiratory physician. A chest CT-scan revealed multiple alveolar opacities with air bronchogram in the lingula, middle lobe, and left lower lobe. Bilateral crackles were present. The patient had never smoked, did not take any medication, had no symptoms of connective tissue disease, and no environmental exposure. C-reactive protein was 38 mg/dL. Hemoglobin was 114 g/L. Leucocytes differential count was normal. Antinuclear antibodies were positive at 1/320 but rheumatoid factor, anti-cyclic citrullinated peptide, anti-double strand DNA and anti-nucleoprotein antibodies were negative. BAL differential count showed 52 % lymphocytes, 6 % neutrophils, and 4 % eosinophils. Cultures were negative. Transbronchial biopsies showed mild chronic interstitial inflammation and intraalveolar fibroblastic buds. Cryptogenic organizing pneumonia was diagnosed. Because of old age, prednisone was started at only 0.5 mg/kg/day (25 mg/day). After 3 days, cough and general symptoms had completely resolved, and dyspnea was markedly reduced. After 2 weeks, chest X-ray was improved. Prednisone was well tolerated and maintained at the same dose for 2 more weeks then tapered over 6 months. The patient was informed about the risk of relapse.
Clinical Features
The clinical features of OP are unspecific and mimic other pulmonary diseases especially infections and malignancies. Many patients initially receive one or more courses of empirical antibiotic therapy, and it is only when this treatment proves ineffective that further investigations are performed. The diagnosis of OP is thus frequently delayed by weeks or even months [2, 14, 18–23].
Disease onset is usually subacute with flu-like symptoms, dry cough, mild dyspnea, fatigue, fever, and weight loss [2, 14, 20, 24]. Productive cough, chest pain, night sweats, arthralgias and myalgias, are less frequent features. Hemoptysis is rare in most large series [23, 25–27], although it has been reported in up to 50 % of cases in one study [28]. Finger clubbing is absent. At chest auscultation, sparse inspiratory crackles are usually heard over the affected areas [26, 27]. Wheezing is uncommon in OP. The frequency of clinical symptoms and signs in a large recent series of OP is summarized in Table 24.1 [27]. No significant difference was found between the clinical presentations of COP and SOP in this series, except for more common crackles in the latter [27]. On rare occasions, OP is incidentally discovered at chest X-ray in an asymptomatic patient [22, 23, 27].
Table 24.1
Frequency of symptoms and signs in organizing pneumonia
No symptoms (incidental finding at chest X-ray) | 6 % |
General | |
Fever | 43 % |
Malaise | 53 % |
Night sweats | 4 % |
Respiratory | |
Cough | 60 % |
Dyspnea | 53 % |
Pleuritic pain | 20 % |
Hemoptysis | 2 % |
Inspiratory crackles | 59 % |
Wheezing | 8 % |
At pulmonary function testing, OP is characterized by mild to moderate restrictive ventilatory defect. Airflow obstruction is found in only a minority of patients, usually smokers [2], and probably reflects preexisting chronic obstructive pulmonary disease unrelated to the OP pathologic process. Carbon monoxide diffusion capacity is usually moderately reduced. Mild to moderate hypoxemia is common [2, 13, 14, 18, 19]. Severe hypoxemia is rare and may result from right-to-left blood shunting through densely consolidated lung parenchyma [29].
Blood cell count usually discloses moderate leucocytosis and neutrophilia [22, 26, 27]. C-reactive protein level and erythrocyte sedimentation rate are usually increased [14, 26, 27, 30]. Bronchoalveolar lavage (BAL) typically shows a mixed pattern alveolitis [14, 20, 21, 23, 24, 31], with predominance of lymphocytes (20–40 %), and a moderate increase of neutrophils (~10 %) and eosinophils (~5 %). Mast cells (~2 %) may be found in one fourth of cases and plasma cells are occasionally present [23]. The lymphocyte CD4/CD8 ratio is usually decreased [14, 21, 23, 24, 31], but it has no specific diagnostic value for OP and is therefore not useful in the diagnostic process. Predominance of eosinophils over lymphocytes is uncommon [31] and suggests the diagnosis of eosinophilic pneumonia rather than OP (cases with overlapping features of eosinophilic pneumonia and OP have occasionally been reported).
Imaging
The imaging characteristics of OP are variable, but can be broadly classified into four patterns: (1) multifocal alveolar opacities, (2) isolated nodule, (3) diffuse infiltrative opacities, and (4) others.
Multifocal Form
The multifocal form is the most typical presentation of OP and accounts for 40–70 % of all cases [22, 23, 26, 32]. It is characterized by multiple bilateral alveolar opacities predominating in the subpleural regions and the lower lung zones, often containing an air bronchogram (Fig. 24.2) [14, 18, 19, 32–34]. A chest computed tomography (CT) is a useful non invasive procedure if OP is suspected, as it often shows more opacities than the chest X-ray, and this multifocal pattern provides an important diagnostic clue for OP. Spontaneous disappearance of some opacities over time and appearance of new infiltrates in other sites occurs in 25–50 % of cases of OP [23, 31], either before treatment or when a relapse occurs (Fig. 24.3). This phenomenon called “migratory opacities” provides another important diagnostic clue for OP, as the differential diagnosis is relatively narrow (Table 24.2). Positron emission tomography has shown a significant fluorodeoxyglucose uptake in OP presenting with parenchymal consolidation [35], but this procedure is not part of the routine assessment of OP. Pleural effusion has usually been reported as uncommon in OP [19, 23], although a small effusion has been found in up to 35 % of cases in one series [28]. A moderate enlargement of mediastinal lymph nodes may be found in about 14 % of cases [36].
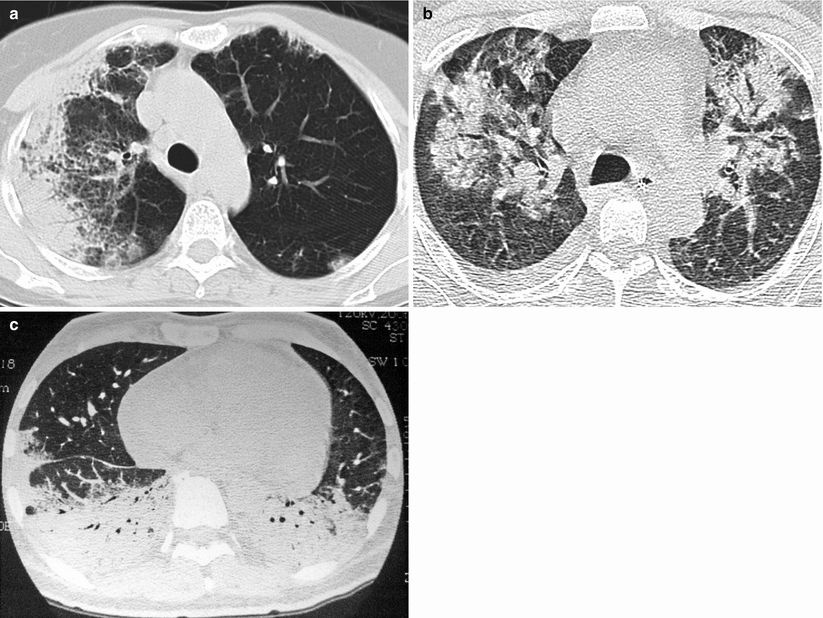
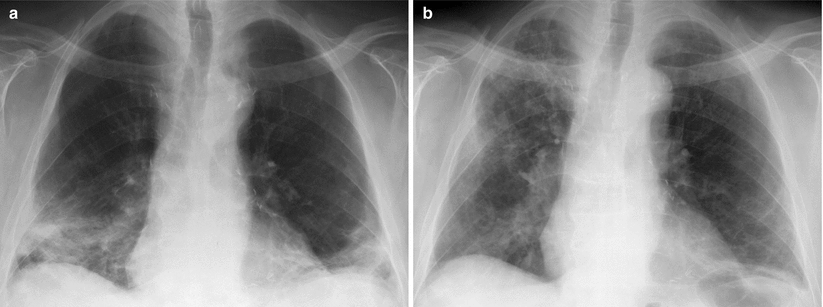

Fig. 24.2
(a–c) Chest CT scan in the classical multifocal form of organizing pneumonia in three patients: multiple bilateral alveolar opacities with an air bronchogram, mainly located in the subpleural areas and the lung bases

Fig. 24.3
Migratory opacities in organizing pneumonia. (a) Bilateral basal subpleural consolidations. (b) Three weeks later, spontaneous healing of right basal consolidation and partial regression of left basal consolidation, but appearance of new ground-glass opacities in the middle and upper fields of the right lung (reproduced with permission of Elsevier from Rev Pneumol Clin 2005;61:193-202)
Table 24.2
Differential diagnosis of migratory pulmonary infiltrates
Organizing pneumonia (cryptogenic or secondary) |
Chronic idiopathic eosinophilic pneumonia |
Secondary eosinophilic pneumonias due to parasitic infections, drug toxicity, etc. |
Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) |
Allergic bronchopulmonary aspergillosis |
Granulomatosis with polyangiitis (Wegener’s) |
Lupus pneumonitis |
Hypersensitivity pneumonitis |
Others (thromboembolic pulmonary manifestations, psittacosis) |
Isolated Nodular Form
This form has been termed “localized”, “solitary”, “nodular”, or “focal” OP, and represents 5–20 % of cases [14, 22, 26]. It appears as a solitary nodule or mass with smooth or irregular margins [14, 37–41] (Fig. 24.4a). In around half of patients, the lesion is found incidentally [39–42].
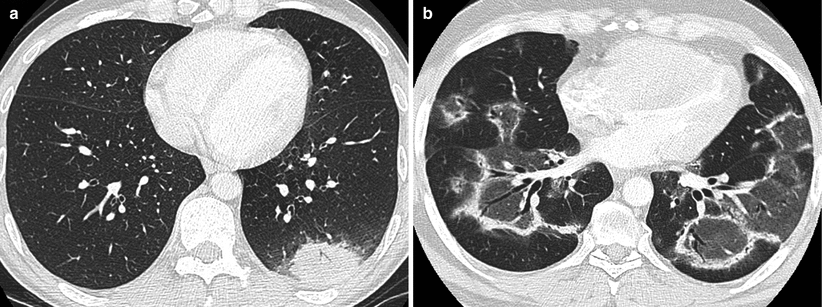

Fig. 24.4
(a) Isolated nodular form of organizing pneumonia: unique dense rounded mass with irregular margins located in the left lower lobe. (b) Reverse halo sign in organizing pneumonia: multifocal opacities characterized by dense margins and central ground glass opacities with air bronchogram. This feature is not specific and may be found in other inflammatory and infectious disorders
In pooled data from five series of nodular OP totalizing 105 cases [39–43], 69 % were men (range across series 56–100 %) and 74 % were smokers or ex-smokers (range 57–72 %, n = 87). Only 47 % were symptomatic (range 17–77 %). A history of recent infection was found in 29 % (range 12–57 %). The upper lobes were affected in 45 % of cases (range 29–58 %, n = 47). The mean size of the nodular opacity was 21 mm (range 6–68 mm). Irregular, lobulated or spiculated margins were present in 72 % (range 54–94 %). An air bronchogram was found in 18 % of cases (n = 47). Satellite nodules were found in 40 % (range 29–56 %, n = 65) and mediastinal lymphadenopathy in 7 % (range 0–19 %, n = 73).
Isolated nodular OP presents with contrast enhancement on CT and positive tracer uptake on positron emission tomography [40, 41], and cannot be confidently distinguished from primary or metastatic malignancy at imaging. This tumor-like appearance frequently leads to surgical resection, and the diagnosis of OP is made retrospectively at pathological examination. In one report, lung resections for isolated nodular OP represented 0.8 % of 1,612 thoracic surgical procedures performed in a 3-year period at one institution [40]. In 105 patients with nodular OP from five series, preoperative transthoracic or transbronchial biopsy was performed in only 23 % of patients (range 0–83 %), whereas 70 % underwent a wedge resection or a segmentectomy (range 17–100), and 7 % had a lobectomy (range 0–24 %). The surgical procedure was curative in most cases without the need for subsequent corticosteroid therapy [40, 41]. Of note, in all of 17 non-operated cases, a spontaneous improvement of the opacity was observed [39, 43]. One practical difficulty in the management of nodular OP is thus to avoid unnecessary lobectomy in this benign disorder mimicking lung cancer. The causes of nodular OP are discussed later in this chapter.
Diffuse Infiltrative Form
A diffuse infiltrative imaging pattern has been reported to occur in 10–40 % of cases in several series of OP [2, 10, 22, 26, 33, 44], some presenting with severe, rapidly progressive disease and respiratory failure [23, 45–51]. Some cases were associated with drugs, connective tissue diseases, or toxic exposure [50–52], whereas other appeared cryptogenic [23, 46, 47, 52].
Diffuse infiltrative OP probably represents a heterogeneous group. It has mainly been reported in early series of OP, suggesting misclassification or overlap with other entities which were unknown at that time. Some early descriptions of diffuse infiltrative OP would probably be now better classified as nonspecific interstitial pneumonia (NSIP), an idiopathic interstitial pneumonia described in the 1990s and characterized histologically by homogeneous chronic interstitial inflammation and/or fibrosis with preserved lung architecture, in which intra-alveolar buds of granulation tissue are a common ancillary finding. Hence, OP pattern representing usually less than 10 % (but sometimes up to 20 %) of the total abnormalities is found in half of cases of NSIP at surgical lung biopsy [53, 54]. Sampling of such focal OP lesions by transbronchial biopsies might thus have led to misdiagnose NSIP as diffuse infiltrative OP. It has also been suggested that a continuum exists between OP and NSIP [54], and that OP/NSIP overlap might explain part of the diffuse infiltrative cases of OP [55]. Hence, patients presenting at imaging with both interstitial changes (histologically corresponding to NSIP) and consolidations (histologically corresponding to OP) have been reported [56]. In a large series of NSIP, the distinction between OP and NSIP has been based upon whether OP pattern represents more or less than 10 or 20 % of the total abnormalities at surgical lung biopsy, an arbitrary criterion [54]. In support of the concept of overlap between OP and NSIP, one study of 22 patients with OP proven by surgical lung biopsy and prolonged CT follow-up reported the evolution of OP consolidations into reticular changes resembling NSIP pattern in a subset of patients [57]. The coexistence of OP and NSIP histological patterns at surgical lung biopsy has been especially observed in idiopathic inflammatory myopathies, in contrast with other autoimmune diseases [58], but more histological data are needed to support the concept of OP/NSIP overlap as a distinct entity.
Other cases diagnosed as diffuse infiltrative OP may actually have had acute interstitial pneumonia, with OP being only a minor histopathological feature or overlapping with DAD at the organizing stage. Other cases could correspond to “acute fibrinous and organizing pneumonia” (AFOP), a recently described entity combining clinical and pathological features of DAD and OP [59] (see below).
Finally, other cases initially reported as diffuse OP may have had acute exacerbation of interstitial lung disease, a recently described acute event occurring in the natural history of idiopathic pulmonary fibrosis, NSIP and other fibrotic interstitial disorders [60]. Acute exacerbations of interstitial lung disease have been associated with histological patterns of either OP or DAD at lung biopsy, the former being associated with a much better short term prognosis [61].
Although genuine diffuse infiltrative OP probably exists, it still awaits better characterization and distinction from similarly appearing entities. Meanwhile, the above-mentioned disorders need to be considered in the differential diagnosis.
Other Imaging Patterns
Rarely, OP may present as multiple, sometimes cavitary nodules [62–65], a micronodular pattern, with multiple small well- or poorly- defined nodules, or nodules with an air bronchogram [66]. Other variants include a bronchocentric pattern, a perilobular pattern resembling thickened interlobular septas, circumferential subpleural linear opacities, and radial opacities [32, 62, 66–69]. A “ring-like”, “reversed halo” or “atoll” pattern has rarely been reported in OP, consisting of a focal round area of ground glass surrounded by a crescent or ring of consolidation (Fig. 24.4b) [66]. Contrary to early beliefs, this sign is not specific to OP and may also be found in Churg-Strauss syndrome, granulomatosis with polyangiitis (GPA, formerly Wegener’s granulomatosis), chronic eosinophilic pneumonia, lymphomatoid granulomatosis, tuberculosis, and various fungal infections [70].
Histopathological Diagnosis of OP Pattern
Buds of granulation tissue (Masson’s bodies) consisting of fibroblasts embedded in a myxoid matrix filling the distal airspaces (alveoli, alveolar ducts, and less commonly distal bronchioles) constitutes the histological hallmark of OP (Fig. 24.1). Associated features include mild interstitial inflammatory infiltrate, type II cell hyperplasia, and intraalveolar foamy macrophages [2, 11, 13]. However, buds of granulation tissue are not specific and may be seen as an ancillary feature in many other disorders such as infections, tumors, pneumonia distal to airway obstruction, hypersensitivity pneumonitis, NSIP, chronic idiopathic eosinophilic pneumonia, or GPA (Wegener’s) [11, 12, 71] (Table 24.3). For instance, OP pattern has been found in the vicinity of tumoral tissue in up to 40 % of resected lung cancers [72]. Thus, a confident histopathological diagnosis of OP pattern requires: (1) the presence of buds of granulation tissue within distal airspaces as the dominant histopathological lesion and not only a minor feature, and (2) the absence of features suggesting another diagnosis such as prominent eosinophilic or neutrophilic inflammation, granulomas, hyaline membranes, acute bronchiolitis, or necrosis (see Box 24.1) [3, 11]. The main differential diagnosis of OP pattern at histopathology includes NSIP and the organizing stage of DAD [3].
Table 24.3
Disorders in which organizing pneumonia pattern may be found as an ancillary histopathological feature
Neoplasms |
Pulmonary infections |
Organization distal to airway obstruction |
Aspiration pneumonia |
Nonspecific interstitial pneumonia |
Hypersensitivity pneumonitis |
Desquamative interstitial pneumonia |
Chronic idiopathic eosinophilic pneumonia |
Secondary eosinophilic pneumonias |
Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) |
Granulomatosis with polyangiitis (Wegener’s) |
Primary pulmonary lymphoma |
Diffuse alveolar damage |
Drug reactions and toxic exposures |
Others |
Box 24.1
Diagnostic Criteria of Organizing Pneumonia
A.
Compatible clinical picture, imaging and bronchoalveolar lavage (see text)
B.
OP pattern at histopathology obtained by transbronchial, transthoracic, or surgical lung biopsy*, showing:
(a)
Presence of intraluminal organizing fibrosis in distal airspaces (bronchioles, alveolar ducts, and alveoli) as the predominant feature, patchy distribution of lesions, uniform temporal appearance, mild chronic interstitial inflammation, and overall preservation of lung architecture
(b)
Absence of other significant abnormalities such as interstitial fibrosis, granulomas, neutrophilic infiltration or abscesses, necrosis, hyaline membranes, prominent airspace fibrin, prominent eosinophilic infiltration, and vasculitis
*Modifying circumstances:
1.
A diagnosis of OP without biopsy is acceptable if a typical clinico-radiological picture and a well-identified cause are present, and if an infectious process has been ruled out
2.
If the patient is too frail or too old for a biopsy, an empirical treatment of corticosteroids may be acceptable, but the risk-benefit ratio of empirical therapy should be carefully weighted in individual cases. Mimics of OP should be ruled out by history and clinical examination, blood and/or urine analyses, and BAL, especially pulmonary infection, drug toxicity, environmental exposure, granulomatosis with polyangiitis (Wegener’s), and lymphoproliferative disorder
3.
If corticosteroids are administered empirically, a critical re-assessment of the diagnosis should be performed after 2–4 weeks. A rapid and complete response to corticosteroids provides an additional argument in favor of OP, although disorders mimicking OP may also initially respond to corticosteroids (see text). Lack of response to corticosteroids after 2–4 weeks should lead to reconsider the initial diagnosis of OP
Adapted from Ref. [3]
Clinicopathological Diagnosis of OP Syndrome
The clinicopathological diagnosis of OP requires the combination of clinical, imaging and histopathological features. Thus, OP is essentially a multidisciplinary diagnosis. BAL is recommended in virtually all cases presenting with multiple or diffuse opacities at imaging in which a diagnosis of OP is suspected. It allows to exclude an active infectious process and to differentiate OP from other inflammatory disorders having a similar picture such as eosinophilic pneumonias. A histological proof of OP should be obtained whenever possible [73]. Transbronchial lung biopsy (TBB) is the most commonly used method, whereas surgical lung biopsy is now performed in a minority of cases, although it can be considered as the gold standard for histological diagnosis of OP.
The diagnostic value of BAL and TBB to diagnose COP has been analyzed in one study [74]. In 37 consecutive patients presenting with clinical features suggestive of COP and bilateral patchy infiltrates at chest X-ray, BAL with >25 % lymphocytes combined with 2 out of 3 other criteria (foamy macrophages >20 %, neutrophils >5 %, or eosinophils >2 % and <25 %) had a sensitivity of 63 % and a specificity of 57 % to diagnose COP [74]. A sensitivity of 20 % and a specificity of 89 % were found in another study using the same criteria [36]. Transbronchial biopsies showing buds of granulation tissue in distal airspaces, chronic inflammation of the alveolar walls, and preserved lung architecture were 64 % sensitive and 86 % specific for the diagnosis of COP [74]. Although generalization of these data is questionable, expert opinion-based current international guidelines consider that if the clinical and imaging picture is typical with multifocal opacities, a TBB showing also typical intraalveolar buds of granulation tissue is sufficient to confidently diagnose OP [3, 55].
If the initial clinical and imaging features are atypical (solitary nodular opacity, diffuse infiltrative pattern) and if an infection or tumor have not been found at bronchoscopy, a video-assisted thoracoscopic surgical lung biopsy is recommended to make sure that OP is the dominant histopathological pattern and not just an ancillary finding in the frame of another pathological process (Fig. 24.5a) [73].
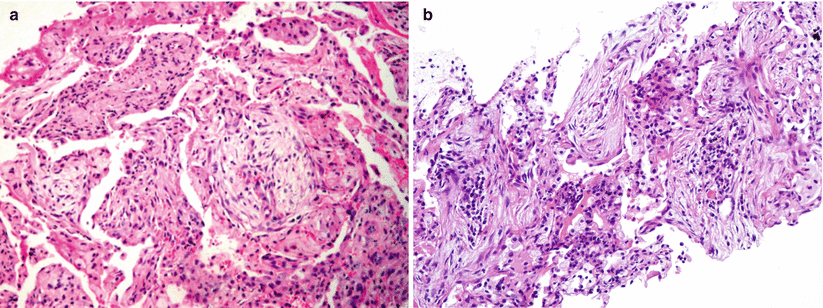

Fig. 24.5
(a) Transbronchial biopsy showing a few buds of granulation tissue filling alveolar spaces, with moderate lymphocytic inflammatory infiltrates of the alveolar walls, in a patient with diffuse parenchymal ground glass opacities. Organizing pneumonia was initially diagnosed, but surgical lung biopsy showed a pattern of nonspecific interstitial pneumonia, in which organizing pneumonia was only an ancillary feature. (b) CT-guided transthoracic needle biopsy in organizing pneumonia. Numerous intraalveolar buds of granulation tissue with fibroblasts and inflammatory cells embedded in a loose myxoid matrix are visible
Transthoracic CT-guided needle biopsy has been recently reported as a useful minimally invasive diagnostic method for OP with a high diagnostic yield [75, 76]. Most patients studied had unilateral or bilateral consolidations or tumor-like lesions, and only a few had a diffuse infiltrative pattern [75, 76]. The most frequent complications were subclinical pneumothorax and minor hemoptysis, occurring in around 30 % of cases. As transthoracic needle biopsy usually provides larger tissue samples than transbronchial biopsy, it may constitute an alternative to surgical lung biopsy in some cases (Fig. 24.5b). However, experience with this technique for the diagnosis of OP is currently insufficient to recommend it for routine clinical use.
Biopsy may be omitted in a minority of cases with typical clinico-radiological and BAL features, and a clearly identified causal agent of OP such as radiotherapy for breast cancer within the past year, recent documented infectious pneumonia, or obvious drug toxicity. In COP, a combination of typical BAL and multiple patchy parenchymal consolidations at imaging has been found diagnostic in half of cases in one series in the absence of a biopsy, and this strategy deserves further studies [36]. If the risk/benefit ratio of lung biopsy is considered unfavorable due to old age, frail patient or significant comorbidities, a presumptive diagnosis of OP and an empirical treatment of prednisone may be an acceptable strategy. However, the disadvantages of prolonged empirical corticosteroid therapy in the absence of a clear diagnosis, and the risk of false diagnosis of OP, should also been kept in mind. Hence, disorders mimicking the clinical and imaging features of OP may initially respond to corticosteroid treatment, including GPA (Wegener’s), primary pulmonary lymphoma, NSIP, or hypersensitivity pneumonitis. Therefore, if the disease follows an unusual course or the response to therapy is inadequate, the diagnosis of OP should be reconsidered, especially if the initial diagnosis was made without biopsy or with transbronchial biopsy only.
Differential Diagnosis
After having assessed the clinical, imaging and histopathological features which make OP a likely diagnostic hypothesis, one must consider other disorders presenting with similar features such as infections, tumors and other inflammatory lung diseases. Imaging could be a starting point to address the differential diagnosis.
In cases presenting with single or multiple areas of parenchymal consolidation, the main differential diagnosis includes infections, minimally invasive or invasive adenocarcinoma (formerly bronchoalveolar carcinoma), eosinophilic pneumonias (either idiopathic or secondary to a known cause), GPA (Wegener’s), Churg-Strauss syndrome, and primary pulmonary lymphoma. The distinction between OP and GPA may be challenging in some cases, as GPA may present with clinical, imaging, and even histological features of OP pattern [11, 71]. Although the latter usually consist of small foci of OP at the vicinity of otherwise typical granulomatous lesions, OP pattern may occasionally be a prominent histological finding in GPA [11, 71].
In patients presenting with a solitary nodule or mass, lung cancer is the main working hypothesis until proven otherwise. When multiple nodules are present, the differential diagnosis includes metastatic tumors, lymphomas, and pulmonary infections including septic emboli.
If OP presents as a diffuse infiltrative disorder at imaging, the differential diagnosis mainly includes hypersensitivity pneumonitis, NSIP, acute interstitial pneumonia, other idiopathic interstitial pneumonias, and acute exacerbation of preexisting interstitial lung disease.
Etiological Diagnosis of OP
The next step in the diagnostic process of OP is to distinguish between SOP and COP. The search for a cause or associated condition should not be overlooked, as removal of an offending agent, such as a drug, is an essential part of therapy. Since there is no clinical, radiological, or histological characteristic allowing to confidently distinguish COP from secondary OP [27], the diagnosis of COP is made by exclusion, when the search for a cause remains negative.
SOP has been associated with numerous causal agents and clinical contexts (Table 24.4) [27, 73]. It frequently occurs in association with various infections mostly caused by bacteria, but occasionally also by viral, fungal, and parasitic agents. Another frequent cause of OP is a drug reaction [73]. A comprehensive and updated list of incriminated drugs is available on www.pneumotox.com. OP can also arise in the context of connective tissue diseases such as idiopathic inflammatory myopathies or rheumatoid arthritis, and in various types of solid cancers and hematologic malignancies, where it should not be mistaken for neoplasm progression or recurrence [77]. One example is provided by bleomycin toxicity: besides diffuse interstitial lung disease, bleomycin can also occasionally induce OP manifesting as pulmonary nodules mimicking metastatic tumor [78–80]. OP can also occur during myelo- or lymphoproliferative syndromes, and after lung or bone marrow transplantation. In the latter, an association has been recently demonstrated between OP and both acute and chronic forms of graft-versus-host disease, suggesting that a causal relationship may exist between these two conditions [81].
Table 24.4
Causes of secondary organizing pneumonia, with relative frequencies of main categories
Infections | ~45 % |
Bacteria (Chlamydia pneumoniae, Coxiella burnetii, Legionella pneumophila, Mycoplasma pneumoniae, Nocardia asteroides, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Streptococcus group B, Streptococcus pneumoniae), virus (herpes virus, human immunodeficiency virus, influenza, parainfluenza, adenovirus, cytomegalovirus, hepatitis C), parasites (Plasmodium vivax), fungi (Cryptococcus neoformans, Penicillium janthinellum, Pneumocystis jiroveci) | |
Drugs | ~20 %
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|