Open Surgical Treatment of Thoracoabdominal Aortic Aneurysms
Hazim J. Safi
Tam T.T. Huynh
Charles C. Miller III
Anthony L. Estrera
Eyal E. Porat
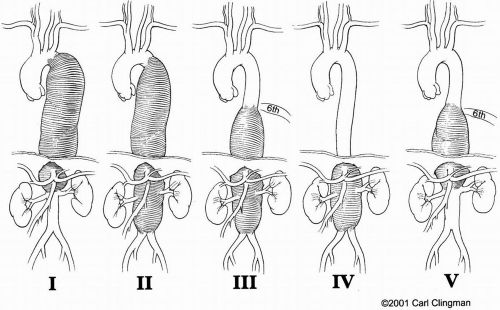
The results of open surgical repair of thoracoabdominal aortic aneurysms (TAAA) vary depending on the extent of the aneurysm (Fig. 14-1) and the surgical approach. Although the incidence of dreaded paraplegia and paraparesis has declined, other major postoperative complications involving the heart, lungs, kidneys, liver, and intestines continue to pose a risk for patients undergoing repair of TAAA, particularly for extent II. The use of adjuncts has, however, greatly improved patient outcome after surgical repair of these extensive aneurysms. In modern-day surgery, a large TAAA with high likelihood of fatal aortic rupture holds a greater threat than the risk of postoperative complications associated with surgical therapy (Fig. 14-2).
Dr. Samuel Etheredge performed the first successful TAAA surgery in 1955, but the operation did not become a commonly
performed procedure until the 1960s when Dr. E. Stanley Crawford introduced what became known as the clamp-and-sew technique. This technique made a remarkable impact on patient survival. However, the operation still had to be done hastily, and surgeons were required to open the chest, clamp the aorta, and sew the graft as quickly as possible, to avoid extended periods of organ ischemia. Consequently, surgeons went searching for ways to provide better organ protection and safely extend the ischemic period of aortic cross-clamp. Early on, surgical adjuncts consisted of pulsatile and nonpulsatile left atrial to femoral bypass. Original studies regarding spinal cord protection included cerebrospinal fluid (CSF) drainage in the early 1960s and monitoring of somatosensory- or motor-evoked potentials in the mid-1980s. Other methods of organ protection have been spinal cooling, systemic hypothermia, and various pharmacologic interventions.
performed procedure until the 1960s when Dr. E. Stanley Crawford introduced what became known as the clamp-and-sew technique. This technique made a remarkable impact on patient survival. However, the operation still had to be done hastily, and surgeons were required to open the chest, clamp the aorta, and sew the graft as quickly as possible, to avoid extended periods of organ ischemia. Consequently, surgeons went searching for ways to provide better organ protection and safely extend the ischemic period of aortic cross-clamp. Early on, surgical adjuncts consisted of pulsatile and nonpulsatile left atrial to femoral bypass. Original studies regarding spinal cord protection included cerebrospinal fluid (CSF) drainage in the early 1960s and monitoring of somatosensory- or motor-evoked potentials in the mid-1980s. Other methods of organ protection have been spinal cooling, systemic hypothermia, and various pharmacologic interventions.
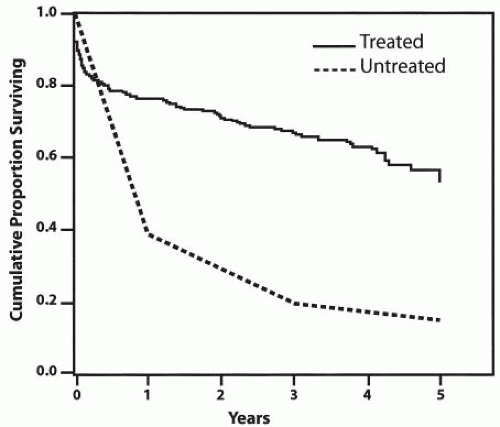 Figure 14-2. Comparison of survival rates for untreated versus surgically treated patients with thoracoabdominal aortic aneurysms. |
In 1992, after several years of animal experiments and promising clinical results reported by ourselves and other investigators, we began using the combined adjunct CSF drainage and distal aortic perfusion for patients undergoing TAAA surgical repair. We then observed considerable improvement in patient outcome. This chapter reviews our experience in the treatment of these complex and extensive aneurysms, with an emphasis on the technical aspects of TAAA surgery; the chapter will also discuss the rationale for our selection of adjuncts.
Etiology
An aortic aneurysm is a localized or diffuse dilatation that exceeds 50% of normal aortic diameter. Arteriosclerosis has long been implicated in the development of aortic aneurysms, but arteriosclerosis primarily involves the intima and typically causes occlusive disease, while aortic aneurysms usually exhibit degeneration. Histologically, aortic aneurysms are characterized by thinning of the media with destruction of smooth muscle cells and elastin, infiltration of inflammatory cells, and neovascularization. Consistently, a chronic inflammatory infiltrate is observed in the vessel wall and consists of macrophages, as well as T and B lymphocytes. The degree of vessel wall inflammation varies, however, and the stimulus for cell migration remains unclear. The inflammatory cells, particularly macrophages, secrete proteases and elastases that can degrade the aortic wall; in turn, the elastin degradation products may act as chemotactic agents for the influx of inflammatory cells. Although the pathogenesis of arteriosclerotic occlusive disease and that of aneurysm disease have been shown to be distinct, the two conditions commonly occur together.
Twenty percent of patients with TAAA have one or more first-degree relatives with the same disease. Marfan syndrome, an inherited connective tissue disorder, is the most common syndrome associated with the formation of aortic aneurysms. Marfan syndrome occurs at a frequency of 1:5000 worldwide and is characterized by skeletal, ocular, and cardiovascular abnormalities. Cardiovascular complications are the major cause of morbidity and mortality in Marfan patients and include thoracic aortic aneurysm and dissection, aortic valve regurgitation, and mitral valve prolapse and regurgitation. Marfan patients tend to develop aneurysms at a much younger age (late 20s to early 30s) than other TAAA patients (>60 years). The genetic defect in Marfan syndrome has been linked to a mutation in fibrillin-1, on chromosome 15, which is inherited in an autosomal dominant manner with high penetrance and clinical variability. However, approximately 25% of patients have Marfan syndrome as the result of a new mutation with no family history. Marfan patients are often considered for surgery at an earlier stage of aneurysm development due to faster rates of aneurysm growth and aortic rupture at smaller diameters. Other known genetic syndromes that predispose individuals to the development of TAAA are Turner syndrome, Ehlers-Danlos syndrome, and polycystic kidney.
In approximately 20% to 40% of patients with aortic dissection, the thoracoabdominal aorta eventually becomes aneurysmal within 2 to 5 years. Conversely, 25% of TAAAs are associated with chronic aortic dissection. Not infrequently, patients may present with acute dissection in a pre-existing aortic aneurysm. Persistent patency of the false lumen in the aorta has been shown to be a significant predictor of aneurysmal formation. However, the presence of chronic aortic dissection or patent false lumen has not been linked to a higher risk of aortic rupture.
A small percentage of TAAAs are related to infection. An infected (mycotic) aneurysm usually results from septic emboli that seed an arteriosclerotic aorta. Another mechanism is contiguous spread, such as from an empyema or adjacent infected lymph nodes. Although any organism can infect the aortic wall, Salmonella, Haemophisis influenzae, Staphylococcus, tuberculosis and Treponema pallidum (syphilis) are most often identified. Infected aortic aneurysms are usually saccular and are thought to be at greater risk for rupture.
Traumatic aortic rupture is a common cause of death from blunt thoracic trauma. In more than 90% of cases, traumatic aortic rupture immediately results in exsanguination and death at the accident scene. Surviving patients generally have a contained rupture, and the aortic transection should be repaired urgently. A small group of patients develop chronic traumatic false aneurysm related to previously unrecognized traumatic aortic transection. False aneurysms are more prone to rupture, and they should be repaired as soon as possible following diagnosis.
Natural History
The incidence of TAAA appears to be on the rise and is currently estimated to be 10.4 cases per 100,000 person-years. The mean age of TAAA patients is between 59 and 69 years old with a male-to-female ratio of three to one. Although the size of an aneurysm is the single most important risk factor for rupture, the rate of growth of an aneurysm has also been shown to predict risk of rupture. The average rate of growth for thoracic aortic aneurysms ranges from 0.10 to 0.45 cm per year, with an exponential growth rate for aneurysms exceeding 5 cm in diameter. Other factors affecting the risk of rupture are gender and age. In general, women develop aortic aneurysms 10 to 15 years later than men. Systemic hypertension has also been shown to increase the risk of rupture, particularly if the diastolic pressure is greater than 100
mm Hg. Also, patients who smoke tobacco or patients with chronic obstructive pulmonary disease (COPD) have been found to be at increased risk of aneurysm rupture. Once an aneurysm has developed, the risk of rupture may be greater in women. The lifetime probability of rupture for any untreated aortic aneurysm is 75% to 80%, but the size at which the aneurysm will rupture and how long it will take to reach that point cannot be easily calculated.
mm Hg. Also, patients who smoke tobacco or patients with chronic obstructive pulmonary disease (COPD) have been found to be at increased risk of aneurysm rupture. Once an aneurysm has developed, the risk of rupture may be greater in women. The lifetime probability of rupture for any untreated aortic aneurysm is 75% to 80%, but the size at which the aneurysm will rupture and how long it will take to reach that point cannot be easily calculated.
Clinical Presentation
Most TAAA patients are asymptomatic, as the condition is often discovered incidentally. Sudden aortic rupture occurs in 10% of patients without prior symptoms. As an aneurysm enlarges it can cause pressure on adjacent structures, leading to discomfort and pain. Affected patients frequently complain of an ill-defined chronic back pain. Pain can also be experienced in the chest, flank, or epigastrium. Pressure on the esophagus can cause dysphagia, pressure on the bronchus can cause dyspnea, and pressure on the recurrent laryngeal nerve can cause hoarseness due to vocal cord paralysis. Direct erosion of the aneurysm into the adjacent tracheobronchial tree or esophagus can result in exsanguination. Paraplegia or paraparesis can occur in patients with TAAA due to acute occlusion of intercostal arteries, usually associated with acute aortic dissection, but can also result from thromboembolism.
Diagnostic Imaging
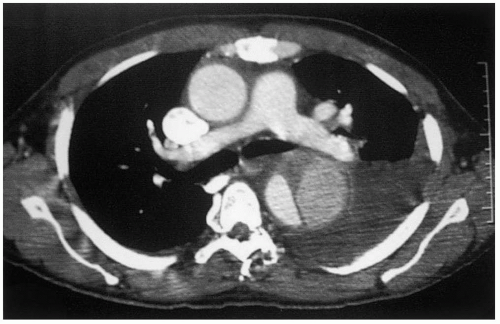
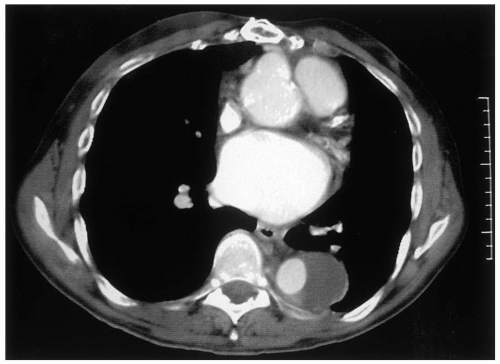
Spiral computed tomography (CT) scan has emerged in the last decade as the diagnostic method of choice for detection of TAAA, replacing aortography as the gold standard. Aortic diameters can be measured serially, from the ascending aorta, arch aorta, and thoracoabdominal aorta at specific levels, to determine the extent of the aneurysm. CT angiography (CTA) acquires images during the arterial phase following a bolus of intravenous contrast. CTA can define the aortic lumen, such as the distinction between the false and true lumen in aortic dissection (Fig. 14-3), and show the presence or absence of thrombus (Fig. 14-4) and inflammatory changes in the aortic wall. The presence of free or contained fluid or blood may indicate aortic rupture. Thinslice CTA image acquisition can also identify patent intercostal arteries. Coronal reformatting or 3-D reconstruction of axial CT images may provide additional views of TAAA but is usually not necessary. A general assessment of other intrathoracic, intra-abdominal, and intrapelvic solid organs can be obtained from CT scan images. CTA is the imaging modality of choice in defining the extent of TAAA and for planning operative strategy. Intravenous iodinated contrast is not essential to determine TAAA size and extent and can be omitted in patients with impaired renal function.
Magnetic resonance imaging (MRI) is a noninvasive modality that has become widely available. Currently, MR angiography (MRA) with gadolinium (Gd) is frequently used as a screening test to detect diseases of the aorta and its branches. The principal advantage of MRA over CTA is that it does not require intravenous iodinated contrast; therefore, it can be performed safely in patients with impaired renal function. Although MRA provides better contrast resolution, its spatial resolution is less precise when compared to spiral CT. In addition, aortic calcification and intramural thrombus are better demonstrated by CT than by MRA. Contraindications for MRA are claustrophobia and internal metallic hardwares (such as pacemakers or orthopedic rods).
Transesophageal echocardiography (TEE) provides an excellent image of the thoracic aorta. TEE can be performed at the bedside or in the operating room. We commonly use TEE when patients are too unstable to be transported to a CT scanner or have impaired renal function. In the operating room, TEE is a great tool for showing aortic wall disease to locate the optimal area for aortic cross-clamping and assessing cardiac function. However, TEE is an invasive modality and requires an experienced operator for optimal visualization and interpretation.
Pre-operative Evaluation
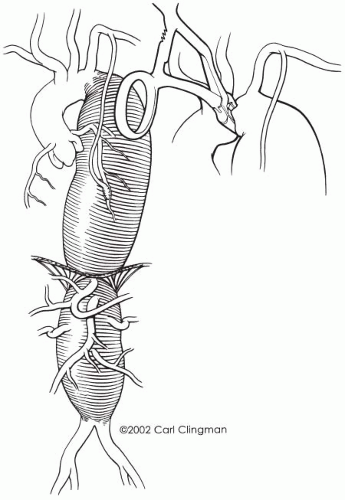
TAAA patients typically have associated diseases that may require intervention, such as carotid endarterectomy, coronary artery angioplasty or bypass, and pulmonary or renal optimization prior to TAAA surgery. A thorough pre-operative cardiac evaluation by an experienced cardiologist is essential. We have found a correlation between a low ejection fraction and poor patient outcome. In general, for patients who require coronary artery stenting, 3 to 4 weeks of platelet inhibition (clopidogrel and aspirin) therapy is maintained to prevent acute in-stent thrombosis. Clopidogrel is stopped 7 days prior to TAAA repair. In patients who have to undergo coronary artery bypass prior to TAAA repair, we specifically avoid using the left internal mammary artery as a conduit, to obviate the possibility of cardiac ischemia in the event that aortic cross-clamping proximal to the left subclavian artery may be required during the TAAA repair (Fig. 14-5). Furthermore, the internal mammary artery may be an important collateral blood supply to the spinal cord. Patients who undergo coronary artery bypass will generally require 4 to 6 weeks to recover before TAAA repair.
Pre-operative consultations with pulmonologists and nephrologists are very helpful. Pre-operative pulmonary rehabilitation, especially breathing exercises and cessation of smoking, can significantly improve patient outcome. Careful evaluation of the patient’s renal function is mandatory, as pre-operative renal insufficiency has been shown to be a predictor of postoperative renal failure, which, in turn, negatively influences mortality rates and the incidence of postoperative neurologic deficits. To minimize preoperative renal injury in patients with suspected chronic renal insufficiency, nephrotoxic agents, such as aminoglycosides, non-steroidal anti-inflammatory medications, and iodinated contrast, may have to be withheld. Pre-operative renal function can also be optimized with good hydration.
Operative Technique
The patient is brought to the operating room and placed in the supine position on the operating table and prepared for surgery. The right radial artery is cannulated for continuous arterial pressure monitoring. General anesthesia is induced. Endotracheal intubation is established using a double lumen tube for selective right lung ventilation during surgery. A sheath is inserted in the internal jugular vein, and a Swan-Ganz catheter is floated into the pulmonary artery for continuous monitoring of the central venous and pulmonary artery pressures. Large-bore central and peripheral venous lines are established for fluid and blood replacement therapy. Temperature probes are placed in the patient’s nasopharynx, rectum, and bladder. Electrodes are attached to the scalp for electroencephalogram (EEG) and along the spinal cord for somatosensory-evoked potential (SSEP) and motor-evoked potential to assess the central nervous system and spinal cord function, respectively. Although a detailed account of the essential anesthetic care during TAAA repair is beyond the scope of this chapter, the importance of adequate maintenance of systemic arterial pressure with judicious blood transfusion cannot be overemphasized, as organ perfusion greatly depends on the systemic circulation.
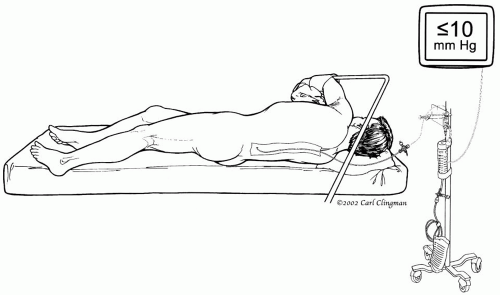 Figure 14-6. Placement of the lumbar catheter in the 3rd or 4th lumbar space to provide cerebrospinal fluid drainage and pressure monitoring |
Cerebrospinal Fluid Drainage
When the descending thoracic aorta is cross-clamped, the spinal cord is quickly rendered ischemic because of the interruption of perfusion to the spinal cord and consequent increased CSF pressure. The rationale for our use of CSF drainage is to increase the spinal cord perfusion pressure directly with distal aortic perfusion and indirectly by reducing CSF pressure. Once all catheters, probes, and lines are in place, we reposition the patient on his or her right side, flexing the knees to open the space between the vertebrae. The anesthesiologist inserts a catheter in the 3rd or 4th lumbar space and advances it for about 5 cm (Fig. 14-6). CSF pressure is kept below 10 mm Hg throughout the surgery and for 3 days postoperatively. Systemic hypotension is avoided during and after surgery to prevent additional hypoperfusion of the spinal cord.
Thoracoabdominal Incision
Once the lumbar catheter is in place, we readjust the patient’s position on the operating table. The right lateral decubitus position
is maintained on a bean bag, and the patient’s shoulders are placed at a right angle to the edge of the table, with the left hip flexed at 60° to allow access to both groins and the left and right femoral arteries. The patient is prepared and draped in the usual sterile fashion. We tailor the incision to fit the extent of the aneurysm (Fig. 14-7). A full thoracoabdominal incision begins between the spine and vertebral border of the left scapula, curves along the 6th rib across the costal cartilage in an oblique line to the umbilicus, and then continues below the umbilicus to just above the symphysis pubis. Resection of the 6th rib facilitates exposure and is routinely performed for all TAAAs except extent IV. Usually, a full thoracoabdominal exploration is necessary for extents II, III, and IV. A modified thoracoabdominal incision begins in the same way as a full thoracoabdominal incision but ends at the costal cartilage or above the umbilicus. A self-retaining retractor placed firmly on the edges of the incision maintains full thoracic and abdominal exposure during the procedure. The left lung is deflated. Mobilization of the aorta begins at the level of the hilum of the lung, cephalad to the proximal descending thoracic aorta. We identify the ligamentum arteriosum and transect it, taking care to avoid injury to the adjacent left recurrent laryngeal nerve. The extent of the distal abdominal aneurysm is assessed. For modified thoracoabdominal exploration, the diaphragm is retracted downward to expose the infradiaphragmatic aorta. When the aortic aneurysm extends below the renal arteries, we continue the full thoracoabdominal exploration below the diaphragm.
is maintained on a bean bag, and the patient’s shoulders are placed at a right angle to the edge of the table, with the left hip flexed at 60° to allow access to both groins and the left and right femoral arteries. The patient is prepared and draped in the usual sterile fashion. We tailor the incision to fit the extent of the aneurysm (Fig. 14-7). A full thoracoabdominal incision begins between the spine and vertebral border of the left scapula, curves along the 6th rib across the costal cartilage in an oblique line to the umbilicus, and then continues below the umbilicus to just above the symphysis pubis. Resection of the 6th rib facilitates exposure and is routinely performed for all TAAAs except extent IV. Usually, a full thoracoabdominal exploration is necessary for extents II, III, and IV. A modified thoracoabdominal incision begins in the same way as a full thoracoabdominal incision but ends at the costal cartilage or above the umbilicus. A self-retaining retractor placed firmly on the edges of the incision maintains full thoracic and abdominal exposure during the procedure. The left lung is deflated. Mobilization of the aorta begins at the level of the hilum of the lung, cephalad to the proximal descending thoracic aorta. We identify the ligamentum arteriosum and transect it, taking care to avoid injury to the adjacent left recurrent laryngeal nerve. The extent of the distal abdominal aneurysm is assessed. For modified thoracoabdominal exploration, the diaphragm is retracted downward to expose the infradiaphragmatic aorta. When the aortic aneurysm extends below the renal arteries, we continue the full thoracoabdominal exploration below the diaphragm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree