The potential of bioresorbable vascular scaffold (BVS) technology has been demonstrated in first-in-man studies with up to 5-year follow-up. This study sought to investigate the 1-year outcomes of the BVS, for the treatment of chronic total occlusions (CTOs), using various imaging techniques. Thirty-five true CTO lesions treated with BVS were included in this prospective study. Scaffolds were deployed after mandatory predilation and intravascular ultrasound analysis. Optical coherence tomography was performed after BVS implantation and at 10 to 12 months. Multislice computed tomography was performed at baseline and at 6 to 8 months. Mean patient age was 61 ± 10 years. The most frequent vessel treated was the right coronary artery (46%). Lesions were classified as intermediate (49%) or difficult/very difficult (26%) according to the Japanese CTO complexity score. Predilation was performed in 100% of lesions, using cutting balloons in 71% of these. The total scaffold length implanted per lesion was of 52 ± 23 mm. All scaffolds were delivered and deployed successfully. Postdilation was undertaken in 63%. By multislice computed tomography at 6 months, we observed 2 cases of asymptomatic scaffold restenosis, subsequently confirmed by angiography. At 12 months, no scaffold thrombosis or major adverse cardiac events were reported. The optical coherence tomography at follow-up showed that 94% of struts were well apposed and covered (5% of uncovered struts and 1% of nonapposed struts), and only 0.6% of struts were nonapposed and uncovered. In conclusion, 1-year results suggest that BVS for CTO is associated with excellent clinical and imaging outcomes. Accurate percutaneous coronary BVS technique might have enabled these promising results.
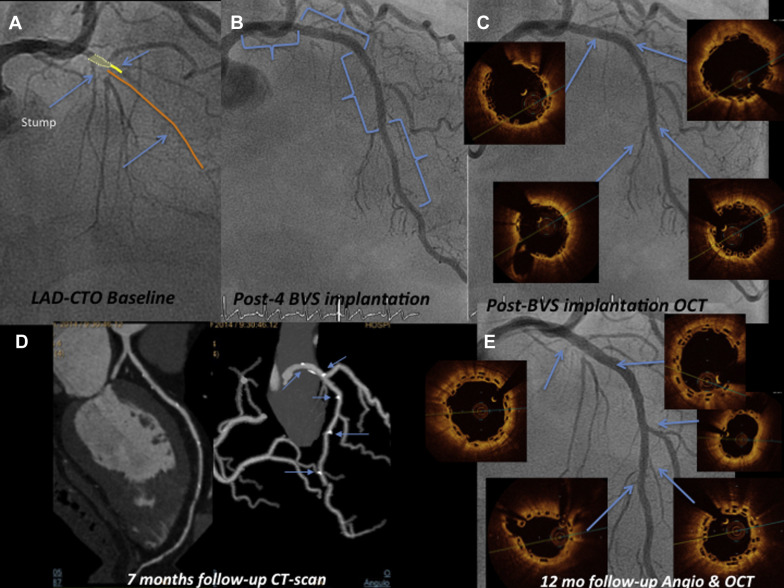
Drug-eluting stents (DES) have been demonstrated to cause less restenosis than bare-metal stents in chronic coronary total occlusion (CTO) recanalization. However, issues about the safety of DES use in CTO that might affect long-term outcome remain unanswered. Hong et al identified CTO revascularization as an independent predictor of DES late-acquired incomplete scaffold apposition (LAISA) with an incidence of 27.5%. In addition, the main risk of LAISA was its association with late stent thrombosis. Thus, a potential advantage of bioresorbable vascular scaffolds (BVSs) over DES is the resolution of this process with stent dismantling and subsequent resorption. In addition, the idea of a “full polymer jacket” with multiple BVS for long CTOs is an appealing one. However, this technology should be noninferior to best-in-class DES. This study was conducted to evaluate the safety and feasibility of using Absorb-BVS for the revascularization of CTOs in a real-world population. Consistent with recent published data, we have reported excellent short-term results using BVS in this setting. In the present report, we describe the 1-year response to BVS in CTOs using multiple imaging techniques, including multislice computed tomography (MSCT) and optical coherence tomography (OCT, Figure 1 ).
Methods
The design of the ABSORB-CTO pilot study has been published previously. In our CTO program, before the procedure MSCT, cardiac magnetic resonance imaging, quality of life, and 6-minute walk test are recommended for all patients. From February 2013 until March 2014, 33 patients with 35 true CTO coronary lesions (EuroCTO definition), with vessel reference diameters between 2.5 and 3.5 mm, were enrolled. Major exclusion criteria were lesions located in the left main, true bifurcation lesions with a side branch ≥2.5 mm in diameter by visual assessment, the presence of any contraindication to implantation of a drug-eluting stent and reference vessel diameters. Angiographic target lesion calcification was not a predefined exclusion criteria but was left to operator’s discretion. From 49 clinically eligible patients with CTO, 14 patients (28.5%) were excluded because of the predefined angiographic criteria (n = 11: calcification and tortuosity lesions [5], true bifurcated lesions with a side branch reference diameter ≥2.5 mm [4], lesions with reference vessel diameter <2.5 mm [n = 1], or ≥4 mm [n = 1]) or because of procedural complications needing bailout stent implantation (n = 3: coronary perforation treated with stent graft, aorto-ostial dissection treated with a 4-mm DES, and distal coronary dissection after BVS implantation treated with 2.25-mm DES).
The second generation of Absorb-BVS comprises a poly-L-lactide backbone coated with a thin layer of a 1:1 mixture of poly-D, L-lactide polymer that contains and controls the release of the antiproliferative drug everolimus. Both poly-L-lactide (backbone) and poly-D, L-lactide (coating) are fully bioresorbable and degrade to lactic acid over 3 years.
Two experienced CTO operators performed all procedures ( Figure 1 ). After crossing the CTO with the guidewire, balloon dilation was performed first with small balloon sizes (1.5 or 2.0 mm). All patients underwent intravascular ultrasound (IVUS) before device implantation with 2 objectives: (a) to analyze the morphologic and anatomical characteristics of the lesion (fibrocalcific or calcified plaques not apparent in previous angiography, areas of inadequate expansion, etc.) and (b) to appropriately size the vessel and avoid scaffold underexpansion. Devices were implanted following the manufacturer’s instructions for use. These recommendations included (a) lesion preparation: predilation of the entire diseased segment was performed either by cutting balloons or noncompliant balloons (balloon:artery ratio = 0.75:1.0) to optimize scaffold apposition and expansion; this was verified by IVUS, before BVS implantation ( Figure 2 ); (b) deployment: scaffolds were deployed by slow inflation (2 atm every 5 seconds) up to 12 to 14 atm; (c) positioning: multiscaffold inplantations were done with minimum overlapping (1 to 2 mm); and (d) postdilation: performed at operator’s discretion but respecting the described expansion limit of the implanted scaffold (0.5 mm more than the nominal scaffold diameter) with a shorter noncompliant balloon. Final postdilation was done with high-pressure non compliant balloon if residual stenosis post-absorb implantation was >20%. When acute procedural success was achieved (final in-scaffold residual stenosis of <30% and Thrombolysis In Myocardial Infarction [TIMI] flow 3 of the target site, without any complications), quantitative coronary angiography (QCA) was performed in 2 orthogonal projections. The result of this optimization was verified by OCT. OCT imaging technique was recommended because is superior in resolution compared with IVUS to verify immediate and long-term results of these new scaffolds.
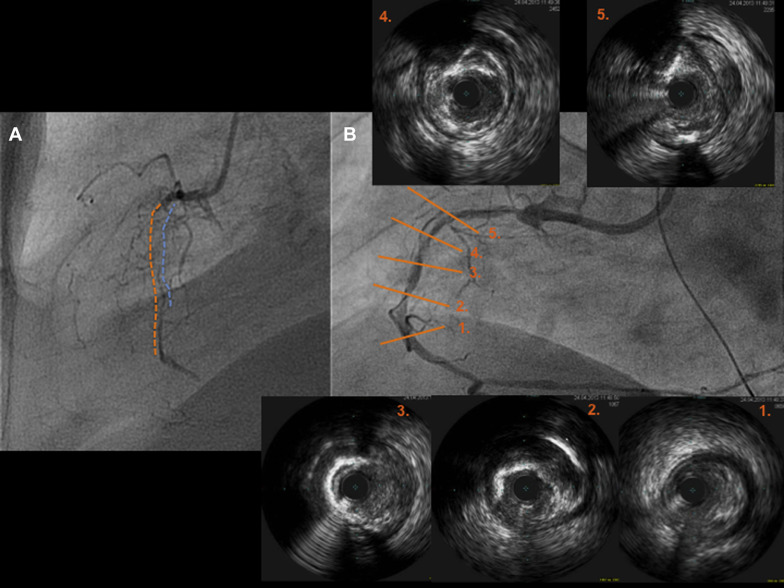
Study definitions are provided in detail elsewhere. In short, according to the EuroCTO Club, “true” CTO was defined as the presence of TIMI 0 flow within the occluded segment with an estimated occlusion duration of >3 months. Acute procedural success was defined by final in-scaffold residual stenosis <30% and TIMI flow 3 in the target vessel. Acute device success was defined as successful delivery and deployment of study scaffolds at the intended target lesion and successful withdrawal of the delivery system, without the need for additional nonstudy stents.
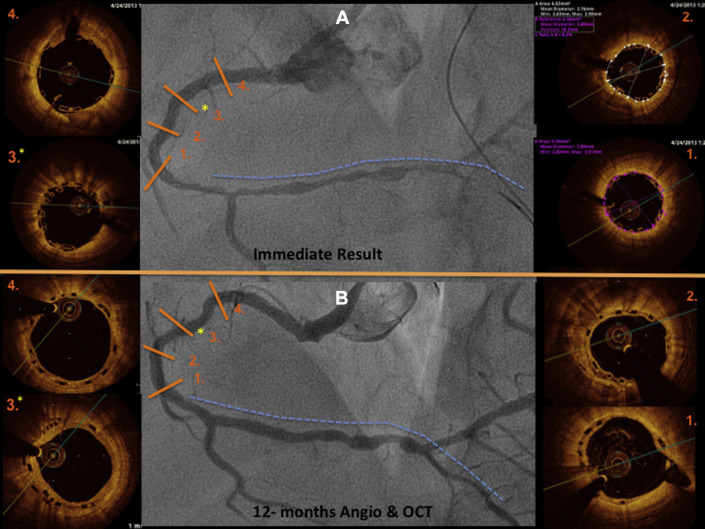
QCA analysis was performed by an independent core laboratory (Barcelona Cardiac Imaging Core-Lab [BARCICORE-lab]) according to standard procedures, using dedicated software (CAAS, version 5.9; Pie Medical BV, Maastricht, The Netherlands). More detailed information on the QCA methodology has been previously described. In each patient, the treated segment and the peri-scaffold segments were analyzed QCA, in paired matched angiographic views after procedure and at 12 months follow-up ( Figures 3–10 ); 32 cases (including restenosis) had 12-month angiographic follow-up. Residual type B coronary dissections were allowed.
OCT was performed (Dragonfly frequency domain OCT C7-XR System; St. Jude Medical, Plymouth, Minnesota) after the procedure and at 12 months. Off-line quantitative and qualitative analyses were performed by an independent core laboratory (BARCICORE-lab) with a dedicated software (St. Jude Medical). In each patient, the scaffold and peri-scaffold segments were analyzed by OCT ( Figures 4, 5, 8–11 ). More detailed information has been previously described. Scaffold incomplete apposition (ISA) was defined as a clear separation between the back (abluminal) side of the strut and the vessel wall with a distance larger than the strut thickness ( Figures 1 and 2, Supplementary data ).
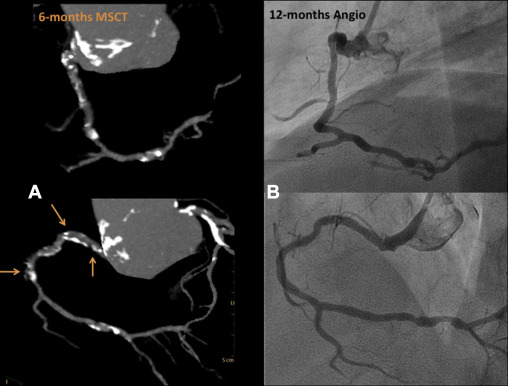
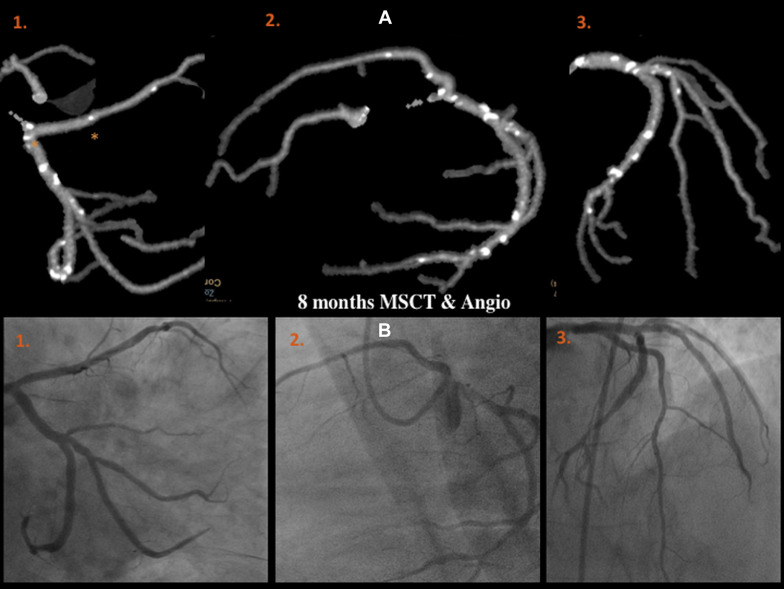
MSCT was performed using a 256-detector Philips Brilliance iCT System (Brilliance iCT; Philips Healthcare, Cleveland, Ohio). Data were analyzed on a dedicated workstation, Brilliance Workspace software (Cardiac Viewer Comprehensive Cardiac Analysis; Philips Healthcare). The scaffold and peri-scaffold segments were analyzed separately and in combination (in-segment analysis). Significant diameter stenosis was defined as ≥50% ( Figures 3, 5–8 , and 10 ).
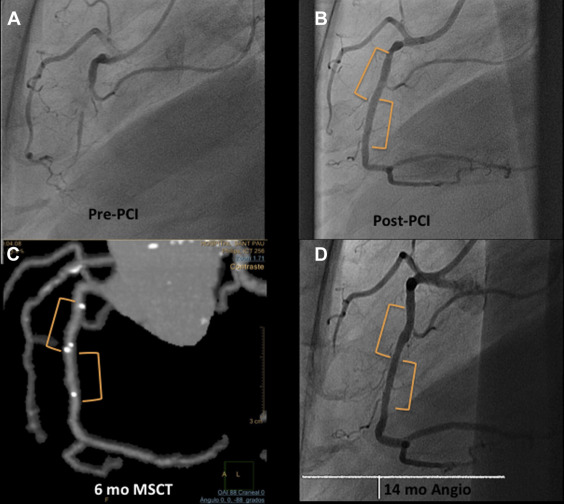
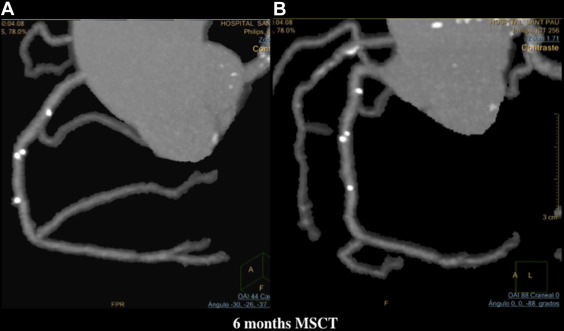

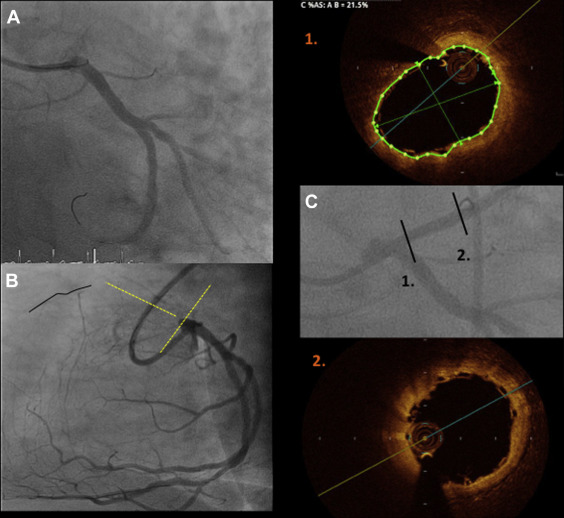
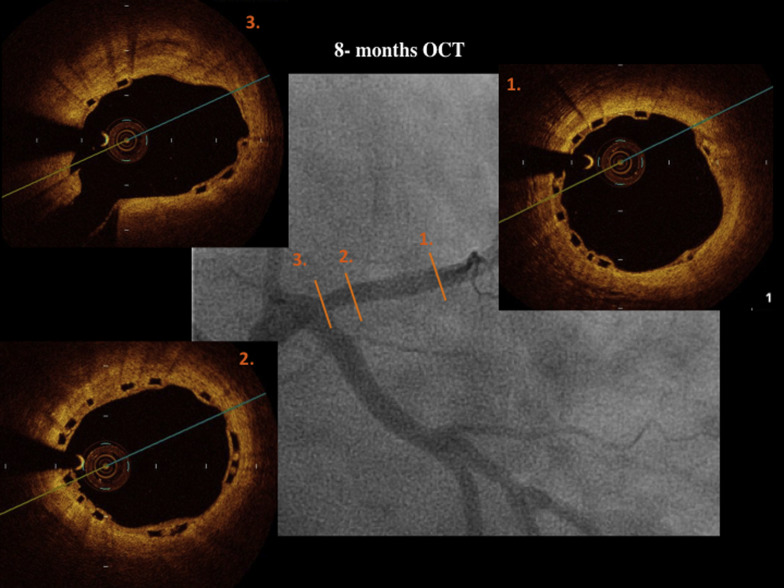
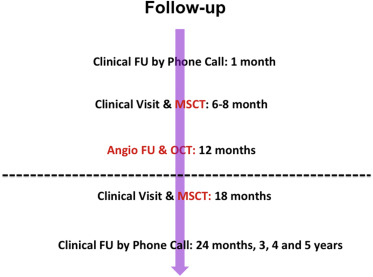
Blood tests for renal function, creatine kinase, and ultrasensitive troponin were performed pre- and at 6, 12, and 24 hours postprocedure. Clinical follow-up was scheduled at 1, 6, 12, and 18 months and 2, 3, 4, and 5 years; OCT was performed after BVS implantation and at 12 months, and MSCT was performed at baseline and at 6 months ( Figure 1 ).
Continuous variables are presented as mean and SD and categorical variables as number and percentage. Comparisons of continuous variables between groups were made using Student’s t test. Categorical variables were compared using either the chi-square or Fisher’s exact test. A p value <0.05 was considered to indicate statistical significance. Statistical analysis was performed using commercially available software (SPSS IBMS 21 for windows; SPPS IBM Inc., Chicago, Illinois).
Results
A total of 35 CTO lesions were prospectively included. Previously published detailed baseline clinical, lesion, and procedural characteristics are listed in Table 1 . In short, this registry comprised a relatively young population (mean age 61 ± 10 years) with stable symptoms of angina or silent ischemia at presentation (85%). After the J-CTO (Multicenter CTO registry of Japan) classification scheme, most lesions were classified as easy (25.6%) or intermediate difficult (48.7%). The decision to take an antegrade or retrograde approach was left to the operator, based on their personal experience and the lesion anatomy using preprocedure CT scan and angiography with simultaneous double injection when applicable. Final antegrade approach was used in 86% (n = 30) of cases, with single-wire technique in 31.4% (n = 11), wire escalation technique in 34.3% (n = 12), and double-wire technique in 20.0% (n = 7). The total number of guidewires used per lesion was 1.8 ± 1.1, being the Fielder XT (ASAHI Intecc Co., Ltd., Aichi, Japan) the most frequent guidewire used in 60% (n = 21). Predilation was undertaken in all cases, by cutting balloon in 71% and rotablator in 8.5%. IVUS analysis revealed subintimal scaffolding present in short segments of 2 complex cases. The total scaffolded length was 52.5 ± 22.8 mm per lesion. Postdilation was undertaken in 63% of lesions. All patients received dual antiplatelet therapy with aspirin and additional clopidogrel (85.7%) or ticagrelor (11.4%) or prasugrel (2.9%).
| Variable | Patients (n=33)/Lesions (n=35) |
|---|---|
| Age (year) (mean +/- SD) | 61 ± 10 |
| Men | 28 (80%) |
| Diabetes Mellitus | 7 (20%) |
| Previous PCI | 13 (37%) |
| Silent Myocardial Ischemia or stable angina pectoris | 30 (86%) |
| ≥ 2 coronary arteries narrowed | 20 (56%) |
| LVEF ≤50% (mean +/- SD) | 8 (23%) |
| Target Vessel: Right Coronary Artery | 16 (46%) |
| CTO location: ostial/proximal | 10 (29%) |
| CTO involving a bifurcated lesion | 7 (20%) |
| In-stent CTO | 2 (6%) |
| Severe CTO calcification | 12 (34%) |
| CTO complexity (J-CTO Score) | |
| Easy (J-CTO score of 0) | 9 (26%) |
| Intermediate (J-CTO score of 1) | 17 (49%) |
| Difficult (J-CTO score of 2) | 3 (9%) |
| Very Difficult (J-CTO score of ≥3) | 6 (17%) |
| Radial or Bi-radial approach | 21 (60%) |
| Pre-dilatation (number of balloon used per lesion) | 2.6 ± 0.97 |
| Cutting Balloon pre-dilatation | 25 (71%) |
| Total scaffold length implanted per lesion (mm) | 53 ± 23 |
| Post-scaffold dilatation (with other non compliant balloon) | 22 (63%) |
Clinical follow-up was completed at 6 and 12 months in 35 and 32 patients, respectively, with no major adverse cardiac events (MACE) or scaffold thrombosis ( Table 2 ). MSCT was performed in all cases at 6 months, with 2 cases of type IV scaffold restenosis per protocol definition. Both were confirmed by angiography at 12 months (n = 2, 6%). Both lesions were mid-circumflex and patients remained asymptomatic; according to patient and family decision, these patients did not undergo repeat revascularization. Table 3 lists the quantitative angiographic results. The mean reference diameter was 2.45 ± 0.46 mm, with a postprocedural residual stenosis of 9.23 ± 17.31%. In-scaffold late loss was −0.28 ± 0.31 mm with a diameter stenosis of 20.77 ± 12.16%.
| Outcomes | In-hospital Patients (n=33)/Lesions (n=35) | 6 month FU Patients (n=33)/Lesions (n=35) | 12 month FU Patients (n=33)/Lesions (n=35) |
|---|---|---|---|
| Acute procedural success | 33 (100%) | ||
| Acute device success | 35 (100%) | ||
| Cardiac Death | 0 | 0 | 0 |
| Q or Non-Q-Myocardial infarction | 0 / 0 | 0 / 0 | 0 / 0 |
| Target lesion revascularization | 0 | 0 | 0 |
| Major Adverse Cardiac Event (MACE) | 0 | 0 | 0 |
| Scaffold thrombosis | 0 | 0 | 0 |
| In- Scaffold restenosis | 0 | 2 (6%) ∗ | 2 (6%) † |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


