The PROMUS Element Plus US Post-Approval Study (PE-Plus PAS) was a prospective, open-label, multicenter, observational study designed to examine outcomes in everyday clinical practice in patients treated with everolimus-eluting, platinum-chromium PROMUS Element Plus stents at 52 centers in the United States. This is the first report of results from this large study. The primary end point of the PE-Plus PAS was 12-month cardiac death or myocardial infarction in the more restricted population of “PLATINUM-like” patients pooled from the PE-Plus PAS, PE-PROVE (PROMUS Element European post-approval study), and PLATINUM Workhorse/Small Vessel trials. Additional clinical end points were tested in the overall PE-Plus PAS patient population. Of the 2,683 patients enrolled in PE-Plus PAS, 70% were men, mean age was 64 years, 33% had diabetes, and 29% were “PLATINUM-like.” Among the PLATINUM-like patients, 12-month cardiac death or myocardial infarction was 1.8% (33 of 1,855) with an upper 1-sided 95% confidence interval of 2.3%, which was significantly less than the prespecified performance goal of 3.2% (p noninferiority <0.001). In the overall PE-Plus population, 12-month target vessel failure (defined as death, MI, or revascularization related to the target vessel) was 6.7% (170 of 2,554), cardiac death was 1.4% (37 of 2,554), MI was 1.1% (28 of 2,554), and ARC-definite/probable stent thrombosis was 0.7% (19 of 2,554). A prespecified secondary end point of 12-month target vessel failure in diabetic patients demonstrated a rate of 4.2% (14 of 332) with an upper 1-sided 95% confidence interval of 6.03%, which was significantly less than the performance goal of 12.6% (p noninferiority <0.001). In conclusion, in this large registry of unselected patients, coronary artery revascularization with the PROMUS Element Plus everolimus-eluting stent demonstrates favorable results with low 1-year clinical event rates.
The PLATINUM clinical trial program evaluated the safety and efficacy of the PROMUS Element Plus everolimus-eluting platinum-chromium coronary stent system (Boston Scientific Corporation, Marlborough, Massachusetts) and supported US and Japanese approval of PROMUS Element. These trials showed that the PROMUS Element stent was noninferior to the predicate cobalt-chromium PROMUS stent, with low rates of clinical events for both stents throughout follow-up. However, the strict enrollment criteria required led to many patients being excluded from the trials, such that those outcomes may not be readily applicable to all patients in routine clinical practice. The PROMUS Element Plus US Post-approval Study (PE-Plus PAS) was a prospective, open-label, observational study. The objectives of this study were to (1) assess safety and efficacy in a broad, unselected patient population representative of everyday clinical practice and (2) compare clinical outcomes to a prespecified performance goal after PROMUS Element percutaneous coronary intervention (PCI) in selected patients from 3 studies.
Methods
The device tested in the PE-Plus PAS was the PROMUS Element Plus everolimus-eluting platinum-chromium coronary stent that incorporates the following key components: the antiproliferative drug everolimus, 2 polymers, and the platinum-chromium alloy Element stent. This stent is coated conformally with 2 layers: a primer layer (poly-n-butyl methacrylate) and a drug matrix layer. The drug matrix layer contains a copolymer of vinylidene fluoride and hexafluoropropylene blended with everolimus (100 μg/cm 2 total weight of drug per unit of stent surface area). This combination of drug, polymer, and platform is identical to the PROMUS Element everolimus-eluting coronary stent (Boston Scientific Corporation). The PROMUS Element Plus stent system differs from the PROMUS Element stent system by incorporating a stent delivery balloon design change (single layer to dual layer), with resultant smaller crossing profile, to enhance flexibility and reduce balloon withdrawal resistance.
PE-Plus PAS was a prospective, open-label, multicenter study designed to collect “real-world” clinical outcome data for patients receiving ≥1 Plusstents as part of PCI for any indication or lesion complexity. Participating physicians enrolled up to 200 patients per center; a patient was considered enrolled once an informed consent form was signed and there was an attempt to implant at least 1 PROMUS Element Plus stent. Per protocol, patients with PROMUS Element Plus implant failures were followed through hospital discharge following the initial attempted index procedure.
Procedures were to be performed according to the PROMUS Element Plus Everolimus-Eluting Platinum Chromium Coronary Stent System Directions for Use, with angiography performed per local standard practice. Staged procedures were allowed when they (1) were planned at the time of the index procedure and (2) occurred within 30 days of the index procedure and the PROMUS Element Plus stent implanted during the staged procedure was implanted in a vessel not previously treated with a PROMUS Element Plus stent as part of this study. Antiplatelet treatment was recommended according to American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines for PCI. Follow-up was scheduled at 30 days, 180 days, and annually through 5 years after study stent implantation.
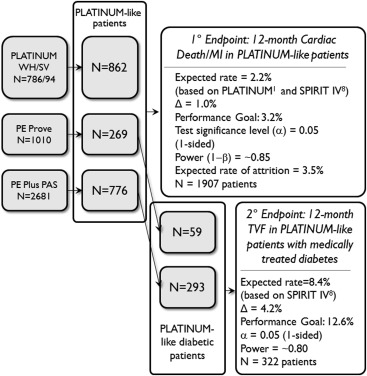
The primary end point of the study was the rate of 12-month cardiac death or myocardial infarction (CD/MI) post-stent implantation in PLATINUM-like patients who received a PROMUS Element Plus stent. PLATINUM-like patients were defined as those without acute MI, graft stenting, chronic total occlusion, in-stent restenosis, failed brachytherapy, bifurcation lesions, ostial lesions, severe tortuosity, moderate or severe calcification by visual estimate in the target lesion or target vessel proximal to target lesion, 3-vessel stenting, cardiogenic shock, left main disease, or acute or chronic renal dysfunction (serum creatinine >2.0 mg/dl or subject on dialysis). PLATINUM-like lesions were defined as follows: (a) lesion length ≤24 mm and diameter ≥2.50 and ≤4.25 mm or (b) lesion length ≤28 mm and diameter ≥2.25 and <2.50 mm. Patients in the primary end point analysis of PE-Plus PAS were pooled from the PE-Plus study, the PLATINUM Workhorse/Small Vessel studies (WH/SV), and the single-arm, European post-approval PE-PROVE (PROMUS Element European post-approval study) registry ( Figure 1 ). Statistical testing was used to determine if the 12-month CD/MI rate in the pooled population met the prespecified performance goal of 3.2% (expected rate of 2.2% plus a delta of 1.0%; based on data from the PLATINUM and the SPIRIT IV trials ). A secondary, statistically powered hypothesis was 12-month target vessel failure (TVF, defined as death, MI, or revascularization related to the target vessel) among PLATINUM-like patients with medically treated diabetes from the PE-Plus and PE-PROVE clinical trials, compared with a performance goal of 12.6% (expected rate of 8.4% + delta of 4.2%; Figure 1 ). The prespecified performance goal for the PLATINUM-like medically treated diabetes subset was based on the SPIRIT IV trial. Additional prespecified end points for the overall PE-Plus population included the incidence of longitudinal stent deformation (LSD) and the overall and PROMUS Element Plus–related rates of cardiac and noncardiac death, MI, target vessel revascularization (TVR), TVF, major adverse cardiac events (MACE, defined as cardiac death, MI, and TVR) and Academic Research Consortium definite/probable stent thrombosis (ST). MI was assessed according to the definition used in the PLATINUM study. Target lesion revascularization (TLR) was defined as stent-related TVR, as assessed by the clinical events committee. For the purposes of the analysis, if it could not be determined with certainty whether MI or death was related to the target vessel, then it was considered TVF by default. Clinical procedural success was defined as post-procedure mean lesion diameter stenosis <30% in 2 near-orthogonal projections with Thrombolysis In Myocardial Infarction 3 flow, as visually assessed by the physician, without the occurrence of inhospital MACE (analyzed per patient). Technical success was defined as successful delivery and deployment of the study stent to the target lesion, without balloon rupture or embolization (analyzed per lesion).
The PE-Plus study complies with the principles of the Declaration of Helsinki and all applicable local and federal regulations. The protocol was reviewed and approved by the Institutional Review Boards/Ethics Committees at each site before patient enrollment, and all patients or their guardians provided written informed consent. The study is registered at the www.clinicaltrials.gov under the identifier NCT01589978 . An independent clinical events committee reviewed and adjudicated all death, MI, TVR, and target vessel ST events according to protocol definitions and for their relation to the PROMUS Element Plus stent. An independent angiographic core laboratory was used to confirm all site-reported cases of LSD; in addition, the core laboratory reviewed all reports of ST for potential LSD. Details of the Clinical Events Committee and the angiographic core laboratory are provided in Supplementary Table 1 .
The statistical methods used in PE-Plus PAS are as follows: The overall sample size was driven by the TVF end point in the PLATINUM-like medically treated diabetic subset. The expected rate of TVF in the PLATINUM-like medically treated diabetic subset was 8.4%, based on results from the SPIRIT IV trial. Given a performance goal of 12.6% (expected rate + delta = 8.4% + 4.2%) and a 1-sided 5% significance level, a minimum of 345 PLATINUM-like medically treated diabetic patients were required to provide ∼80% power for TVF at 12 months. Per protocol, this required that the sample size comprised PLATINUM-like medically treated diabetic patients from the PE-PROVE study and the PE-Plus PAS. Assuming an attrition rate of no more than 3.5% at 12 months, 57 PLATINUM-like medically treated diabetic patients from PE-PROVE were projected to be available for TVF end point analysis. As a result, 288 PLATINUM-like medically treated diabetic patients were needed from the PE-Plus PAS to complete a total enrollment of 345 patients (n = 345 − 57 = 288). Based on the TAXUS Libertē post-approval study, approximately 11.1% of those enrolled in PE-Plus were expected to be PLATINUM-like medically treated patients with diabetes; thus 2,595 total patients were required at 12 months to yield ∼80% power in this prespecified diabetic subset. Assuming an attrition rate of no more than 3.5% in both the PLATINUM-like and PLATINUM-like medically treated diabetic subsets, a total enrollment of approximately 2,689 patients was required in the PE-Plus study. For the primary statistical hypothesis, the expected 12-month CD/MI rate was estimated at 2.2% based on data from the PLATINUM and the SPIRIT IV trials. The performance goal is 3.2% using a delta of 1.0%. Given the performance goal and a 1-sided 5% significance level, approximately 1,706 PLATINUM-like patients were needed to provide at least 80% power to reject the null hypothesis.
Clinical outcomes were evaluated as binary % or as Kaplan-Meier estimates with standard error from Greenwood’s formula. Discrete variables were expressed as % (count/n) and continuous variables were expressed as mean ± SD (n). The primary end point and key secondary end point of TVF in PLATINUM-like medically treated patients with diabetes were assessed using a 1-sided, single binomial test to compare the observed rate against the performance goal and using the normal approximation of the test statistic. The performance goal was considered as met if the 1-sided upper 95% confidence bound for the observed binary rate was less than the performance goal. All analyses were conducted using SAS, version 9.0 or greater (SAS Institute, Cary, North Carolina).
Results
A total of 2,683 patients were enrolled at 52 clinical sites in the United States ( Supplementary Table 2 ). Of these, 2 patients did not have a stent implanted because of inability to cross the lesion and, per protocol, were ineligible for inclusion in the 12-month analysis. These 2 patients did not have any MACE through discharge.
Of the remaining 2,681 patients, 776 (29%) were considered PLATINUM like ( Figure 2 ). At 1 year, 95% (2,535 of 2,681) of the patients had 12-month clinical follow-up available or had died; the predominant reason for lack of follow-up was a missed 12-month visit (n = 104).
Baseline patient and angiographic characteristics are listed in Tables 1 and 2 , respectively; procedural characteristics are provided in Table 3 . Clinical procedural success was 99%, and technical success was 99.9%. At hospital discharge, 96% of patients were on dual antiplatelet therapy, with 91% continuing dual antiplatelet therapy through 1 year ( Table 4 ).
| Variable | PE-Plus – Overall (N=2,681) |
|---|---|
| Men | 69.9% (1874/2,681) |
| Age (years) | 63.7±11.1 (2,681) (28.0, 91.0) |
| Current Smoker | 22.8% (610/2,681) |
| Medically Treated Diabetes Mellitus | 32.9% (882/2,681) |
| Insulin-Requiring | 13.8% (371/2,681) |
| Hyperlipidemia Requiring Medication | 75.2% (2,016/2,681) |
| Hypertension Requiring Medication | 77.5% (2,077/2,681) |
| Prior Cerebrovascular Accidents | 4.2% (113/2,681) |
| Renal Disease | 10.6% (283/2,681) |
| Peripheral Vascular Disease | 11.2% (299/2,681) |
| Family History of Coronary Artery Disease | 60.6% (1,625/2,681) |
| Prior Myocardial Infarction | 52.6% (1,409/2,681) |
| Congestive Heart Failure | 10.3% (275/2,681) |
| NYHA Classification | |
| I | 2.2% (58/2,681) |
| II | 2.9% (79/2,681) |
| III | 1.8% (49/2,681) |
| IV | 0.4% (12/2,681) |
| Stable Angina Pectoris | 23.1% (620/2,681) |
| Unstable Angina Pectoris | 50.2% (1,347/2,681) |
| Silent Myocardial Ischemia | 5.6% (149/2,681) |
| Prior Percutaneous Coronary Intervention | 43.2% (1,159/2,681) |
| Prior Coronary Artery Bypass Graft | 17.0% (457/2,681) |
| Left ventricular ejection fraction Measured | 86.9% (2,331/2,681) |
| Left ventricular ejection fraction (%) | 53.711.2 (2,286) (5.0, 85.0) |
| Left ventricular ejection fraction ≤30% | 5.7% (131/2,286) |
| Event | N=3,605 Lesions |
|---|---|
| De Novo Lesion | 90.3% (3,255/3,605) |
| Culprit Lesion for STEMI | 11.8% (426/3,605) |
| In-stent Restenosis (DES) | 5.1% (183/3,605) |
| In-stent Restenosis (BMS) | 2.8% (102/3,605) |
| Ostial Lesion | 4.6% (165/3,605) |
| Bifurcation Lesion | 8.0% (288/3,605) |
| Chronic Total Occlusion | 5.2% (187/3,605) |
| Thrombus Present | 12.3% (442/3,605) |
| Reference Vessel Diameter (mm) | 2.94 ± 0.51 (3,571) |
| Lesion Length (mm) | 17.0 ± 10.3 (3,571) |
| Preprocedure %DS | 86.7 ± 10.7 (3,604) |
| Moderate Vessel Tortuosity | 10.0% (362/3,605) |
| Severe Vessel Tortuosity | 4.1% (149/3,605) |
| Moderate or Severe Lesion Calcification | 14.1% (509/3,605) |
| Location of Coronary Narrowing | |
| Right | 32.3% (1,165/3,605) |
| Left Anterior Descending | 37.1% (1,339/3,605) |
| Left Circumflex | 23.5% (848/3,605) |
| Left Main | 1.5% (54/3,605) |
| Bypass graft | 5.5% (199/3,605) |
| Event | N=2,681 Patients N=2,712 Procedures N=3,605 Lesions N=4,021 Stents |
|---|---|
| Emergent/Urgent Procedure | 41.0% (1,113/2,712) |
| Number of Lesions Treated Per Patient | 1.3 ± 0.6 (2,681) |
| ≥3 Lesions | 6.2% (166/2,681) |
| Number of Vessels Treated Per Patient | 1.1 ± 0.4 (2,681) |
| ≥3 Vessels | 0.8% (21/2,681) |
| Stents Implanted Per Patient | 1.5 ± 0.8 (2,681) |
| Stent Length (mm) | 19.5 ± 7.7 (4,019) |
| Stent Diameter (mm) | 2.87 ± 0.48 (4,021) |
| Stent Deployment Pressure (atm) | 14.1 ± 3.5 (4,014) |
| Post-dilation Performed | 55.6% (2,005/3,605) |
| Post-Procedure Target Lesion %DS | 1.0 ± 5.6 (3,605) |
| Post-procedure TIMI flow 3 | 99.4% (3,583/3,605) |
| Clinical Procedural Success (Patient-based) | 98.6% (2,644/2,681) |
| Technical Success (Lesion-based) | 99.9% (3,605/3,609) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


