Coronary artery calcium (CAC) is associated with poor angiographic results and higher rates of complications after percutaneous coronary intervention (PCI). Limited data are available regarding the impact of angiographically evident CAC on long-term outcomes after primary PCI in patients presenting with ST-segment elevation myocardial infarction (STEMI). In this single-center, registry-based retrospective cohort analysis, we analyzed 2,143 consecutive patients presenting with STEMI who underwent primary PCI within 12 hours of symptom onset. Patients were divided based on degree of CAC (determined by visual inspection of angiograms) as follows: (1) moderate-to-severe CAC (n = 306; 14.3%) and (2) minimal-to-none CAC (n = 1,837; 85.7%). The primary end point was all-cause mortality at 1-year after PCI. Patients with moderate-to-severe CAC were older, women, and had higher rates of hypertension, chronic kidney disease, and peripheral vascular disease. Moderate-to-severe CAC was associated with higher rates of anterior myocardial infarction, advanced Killip class, and poor final angiographic results. At 1-year follow-up, rates of all-cause mortality were higher in the moderate-to-severe CAC cohort than those in the minimal-to-none CAC cohort (8.5% vs 4.7%; p = 0.008). However, after accounting for major clinical and angiographic characteristics, moderate-to-severe CAC on presenting STEMI angiogram was no longer predictive of 1-year all-cause mortality. In conclusion, advanced CAC burden occurs in ∼15% of patients undergoing primary PCI for STEMI and reflects a marker of adverse prognosis late into follow-up after PCI.
Coronary artery calcium (CAC) is commonly encountered by operators during elective or nonelective percutaneous coronary intervention (PCI) and poses distinct challenges to early and late periprocedural management. CAC has been associated with adverse angiographic results and poor post-PCI outcomes in stable patients undergoing elective PCI. Limited data however exist regarding the influence of CAC on prognosis in patients undergoing emergent or urgent PCI for acute coronary syndromes (ACS), specifically ST-segment elevation myocardial infarction (STEMI). As such, the assessment of the real-world impact of CAC in the background of contemporary PCI practice is warranted. The objective of the present study was to assess the association between varying degrees of CAC and 1-year outcomes after primary PCI in patients presenting with STEMI in a large, single-center registry.
Methods
This single-center, registry–based study included 2,143 consecutive unselected patients presenting with STEMI who were treated with primary PCI at Rabin Medical Center, Petah Tikva, Israel from July 2001 to July 2014. This post hoc study was performed using data from an ongoing prospective institutional registry of patients undergoing PCI for various indications that has been established to collect and monitor patient-related data and clinical events. Patients presenting with cardiogenic shock were excluded. All subjects provided explicit written informed consent before cardiac catheterization, and the study was approved by the Investigational Review Board of Rabin Medical Center. Baseline characteristics, procedural details, and quantitative coronary angiographic data were collected and available for all study participants.
All patients received aspirin and unfractionated heparin (70 U/kg) before cardiac catheterization. Clopidogrel (300 or 600 mg) or prasugrel 60 mg was administered as a loading dose before or immediately after PCI. Utilization of glycoprotein IIb/IIIa inhibitors and choice of drug-eluting stent (DES) versus bare-metal stent (BMS) were left to the discretion of the primary operator. All stents were implanted with moderate-to-high deployment pressure (14 to 18 atm). All patients received dual antiplatelet therapy with aspirin 100 mg daily and a thienopyridine (clopidogrel or prasugrel) for at least 12 months after PCI.
Coronary angiograms were analyzed at the dedicated institutional core angiographic laboratory by experienced cardiologists blinded to clinical outcomes, using the MDViewTM QA System (Medcon Telemedicine Technology McKesson, Tel Aviv, Israel). All angiograms were evaluated at baseline and after PCI. The contrast-filled guiding catheter (6Fr or 7Fr) was used as a calibration standard. Reference and minimal lumen diameter were assessed before and after PCI. Standard morphologic criteria were used to determine lesion location, lumen diameter, stent length, and thrombus size. Percentage diameter stenosis and Thrombolysis In Myocardial Infarction flow grade (0 to 3) were determined before and after PCI. Procedural failure was defined as final diameter stenosis >30% or postdilatation Thrombolysis In Myocardial Infarction flow <3.
CAC was determined by the angiographic core laboratory from the pre-PCI coronary angiograms. Moderate CAC was defined as radiopaque densities noted only during the cardiac cycle and typically involving only 1 side of the vascular wall, and severe CAC was defined as radiopaque densities noted without cardiac motion before contrast injection and generally involving both sides of the arterial wall.
Immediate and in-hospital events were recorded in the institutional database. Clinical events were prospectively collected in the institutional database. Patients completed standardized questionnaires for clinical events either by telephone or in the outpatient clinics at 6-month intervals. When indicated, records from peripheral hospitals were acquired to verify the events in the follow-up period. Survival status at follow-up was assessed by municipal civil registries at 1 and 2 years. All events were further confirmed and adjudicated by the institutional clinical events adjudication committee.
The primary end point of the study was all-cause mortality at 1 year. Although detailed follow-up was pursued through 24 months, 1 year was selected a priori for the primary multivariate analyses given ∼95% of patients had complete follow-up data at this interval. The secondary end points were incidence of definite stent thrombosis (ST), recurrent nonfatal myocardial infarction (MI), coronary artery bypass graft surgery (CABG), ischemia-driven target vessel revascularization (TVR), and major adverse cardiac events (MACE) after 2 years of index PCI. ST was defined by Academic Research Consortium criteria in the background of ACS and/or reinfarction in the culprit coronary territory with angiographically proven thrombosis of the previously implanted stent. Recurrent nonfatal MI was defined as an episode of chest discomfort suggestive of ACS and an elevation in serum cardiac enzymes levels to at least twice the upper limit of normal or the appearance of 1 or more new pathologic Q waves after index hospitalization. Ischemia-driven TVR was defined as any revascularization that involved the target vessel. MACE was defined as a composite of cardiac death, recurrent nonfatal MI, and ischemia-driven TVR.
Continuous data are summarized as mean ± SD or median (interquartile range) and were compared using Student t tests or analyses of variance testing. Categorical variables are presented as frequency (%) and were compared by the chi-square tests or Fisher’s exact tests. Normality of variable distributions was assessed using Kolmogorov–Smirnov testing. Time-to-event curves were constructed using the Kaplan–Meier method and compared using log-rank testing. Logistic regression analyses were performed to identify independent predictors of the primary end point. Effect sizes are presented as odds ratios and 95% CIs. Step-wise variable selection of significant univariate predictors (p <0.1) was used to identify variables to be subsequently included in the multivariate model. Multivariate logistic regression analyses were performed to determine independent predictors of the primary end point, accounting for known baseline cardiovascular risk differences. All statistical analyses were performed with STATISCA software (StatSoft Inc., Tulsa, Oklahoma). p Value <0.05 was considered statistically significant.
Results
A total of 2,143 consecutive patients with STEMI were included in the final analysis. Of the study cohort, 306 patients (14.3%) had moderate/severe CAC, whereas 1,837 patients (85.7%) had none/minimal CAC.
Patients with moderate/severe CAC were older, women, had higher rates of hypertension, chronic kidney disease (CKD), peripheral vascular disease, and statin use ( Table 1 ). These patients were also more likely to present with anterior MIs, higher Killip classes (>1), higher Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications scores, and lower left ventricular ejection fractions. Rates of current smoking and family history of coronary artery disease were higher in patients with none/minimal CAC.
| Variable | Coronary Calcium | P | |
|---|---|---|---|
| Moderate or Severe (n=306) | Minimal to None (n=1837) | ||
| Age (years, mean±SD) | 68.2±12.6 | 59.3±12 | 0.001 |
| Men | 74.0% | 84.0% | 0.001 |
| Current smoker | 32.0% | 46.0% | <0.001 |
| Diabetes mellitus | 30.0% | 25.0% | 0.08 |
| Hyperlipidemia | 54.0% | 52.0% | 0.5 |
| Hypertension | 65.0% | 49.0% | <0.001 |
| Chronic kidney disease (estimated glomerular filtration rate<60 mL/min/1.73 m 2 ) | 22.0% | 11.0% | <0.001 |
| Peripheral vascular disease | 7.6% | 4.1% | 0.01 |
| Family history of coronary artery disease | 23.0% | 35.0% | 0.003 |
| Prior coronary bypass | 2.6% | 3.0% | 0.7 |
| Prior percutaneous coronary intervention | 17.0% | 16.0% | 0.7 |
| Prior myocardial infarction | 15.0% | 14.0% | 0.7 |
| Prior cerebrovascular accident | 7.9% | 5.5% | 0.1 |
| Baseline medication utilization | |||
| Aspirin | 93.0% | 92.0% | 0.8 |
| Beta-blockers | 7.3% | 7.3% | 1 |
| Angiotensin converting enzyme inhibitors | 6.0% | 6.0% | 1 |
| Statins | 45.0% | 38.0% | 0.03 |
| ST-segment elevation myocardial infarction presentation | |||
| Anterior myocardial infarction | 61.0% | 43.0% | <0.001 |
| CADILLAC score | 6.1±3.9 | 3.8±3.3 | <0.001 |
| Killip class>1 | 17.0% | 11.0% | 0.001 |
| Systolic blood pressure (mm Hg, mean±SD) | 142±25 | 137±25 | 0.01 |
| Door to needle time (hours) | |||
| Mean±SD | 1.8±3.0 | 1.6±2.3 | 0.1 |
| Median (IQR) | 1[1-2] | 1[0.7-1.5] | 0.001 |
| Time from symptom onset to emergency department (hours) | |||
| Mean±SD | 3.4±2.7 | 3±2.5 | 0.01 |
| Median (IQR) | 3[1.5-4] | 2[1-4] | 0.01 |
| Clopidogrel or prasugrel load | 66.0% | 65.0% | 0.9 |
| Culprit coronary artery | |||
| Left anterior descending | 61.0% | 42.0% | <0.001 |
| Left main | 0.3% | 0.5% | |
| Left circumflex | 7.2% | 16.5% | |
| Right | 29.0% | 37.0% | |
| Diagonal branch | 2.0% | 2.5% | |
| Saphenous vein graft | 0.3% | 1.4% | |
| 2- or 3-vessel coronary disease | 70.0% | 57.0% | <0.001 |
| Thrombotic lesion | 66.0% | 82.0% | <0.001 |
| Pre-TIMI grade 0/1 flow | 58.0% | 63.0% | 0.08 |
| Post-TIMI grade 3 flow | 90.5% | 97.0% | <0.001 |
| Pre-stent stenosis (%, mean±SD) | 96±6 | 96±7 | 1 |
| Post-stent stenosis (%, mean±SD) | 7±22 | 3±11 | <0.001 |
| Lesion length (mm, mean±SD) | 17.2±8.8 | 16±22 | 0.002 |
| Minimal lumen diameter (mm, mean±SD) | 0.1±0.2 | 0.1±0.2 | 1 |
| Reference vessel diameter (mm, mean±SD) | 3.0±0.5 | 3.1±0.5 | 0.001 |
| Stent placed | 94.0% | 97.0% | 0.005 |
| Drug-eluting stent (vs. bare metal stent) | 22.0% | 22.0% | 1 |
| Stent diameter (mm, mean±SD) | 3.0±0.4 | 3.1±0.5 | 0.005 |
| Pressure at stent deployment (atm, mean±SD) | 18±3 | 17±3 | 0.001 |
| Procedural technical success | 90.5% | 97.0% | <0.001 |
| No-reflow phenomenon | 7.4% | 4.7% | 0.05 |
| Left ventricular ejection fraction (based on echocardiography, %, mean±SD) | 39±10 | 43±10 | <0.001 |
| Left ventricular ejection fraction <40% (based on echocardiography) | 56.0% | 39.0% | 0.005 |
The left anterior descending coronary artery was more frequently the culprit vessel in patients with moderate/severe CAC compared with none/minimal CAC group ( Table 1 ). Patients with moderate/severe CAC were more likely to have multivessel coronary disease, longer lesions, smaller vessel sizes, and required higher stent inflation pressures during PCI. Patients with moderate/severe CAC were more likely to have suboptimal angiographic results after PCI, with greater residual stenoses, slow flow, or no reflow.
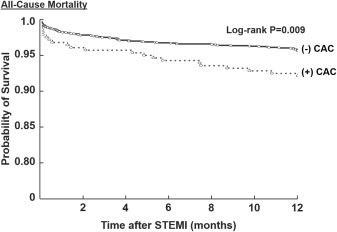
Outcomes during 2 years of follow-up are listed in Table 2 . Of the 2,143 patients with STEMI, complete clinical outcome data were available in 2,084 patients (97.2%) at 6 months, 2,025 (94.5%) at 1 year, and 1,903 (88.8%) at 2 years. The rates of the primary end point of all-cause mortality at 1 year was significantly higher in the moderate/severe CAC group than those in the none/minimal CAC group (8.5% vs 4.7%; p = 0.008). Figure 1 shows the significant unadjusted survival difference between the moderate/severe CAC and none/minimal CAC groups (log-rank p = 0.009). Similarly, the incidence of all-cause mortality at 24-month follow-up was significantly higher in the moderate/severe CAC group compared with the none/minimal CAC group (13.6% vs 6.8%; p = 0.001). However, after adjusting for variable baseline risk profiles between the 2 groups, the presence of moderate/severe CAC was not an independent predictor of all-cause mortality at 1 year (adjusted odds ratio 1.2, 95% CI 0.4 to 1.3; p = 0.3; Table 3 ).
| Coronary Calcium | P | ||
|---|---|---|---|
| Moderate or Severe | Minimal to None | ||
| 1-month | n=306 | n=1837 | |
| Death | 3.9% | 2.1% | 0.06 |
| Definite stent thrombosis | 2.3% | 1.4% | 0.2 |
| Recurrent myocardial infarction | 2.3% | 2.2% | 0.9 |
| Coronary artery bypass graft surgery | 2.9% | 1.2% | 0.02 |
| Target vessel vascularization | 3.9% | 2.0% | 0.03 |
| Major adverse cardiovascular events | 9.5% | 5.2% | 0.002 |
| 6-months | n=297 | n=1787 | |
| Death | 6.1% | 3.6% | 0.04 |
| Definite stent thrombosis | 3.4% | 2.2% | 0.2 |
| Recurrent myocardial infarction | 4.8% | 4.2% | 0.7 |
| Coronary artery bypass graft surgery | 6.1% | 3.0% | 0.005 |
| Target vessel vascularization | 9.9% | 7.9% | 0.3 |
| Major adverse cardiovascular events | 17.8% | 13.2% | 0.03 |
| 12-months | n=284 | n=1741 | |
| Death | 8.5% | 4.7% | 0.008 |
| Definite stent thrombosis | 3.6% | 2.2% | 0.2 |
| Recurrent myocardial infarction | 5.7% | 5.1% | 0.6 |
| Coronary artery bypass graft surgery | 7.2% | 3.5% | 0.003 |
| Target vessel vascularization | 12.5% | 11.0% | 0.5 |
| Major adverse cardiovascular events | 23.7% | 17.5% | 0.01 |
| 24-months | n=265 | n=1638 | |
| Death | 13.6% | 6.8% | 0.001 |
| Definite stent thrombosis | 4.3% | 3.2% | 0.3 |
| Recurrent myocardial infarction | 7.3% | 6.3% | 0.5 |
| Coronary artery bypass graft surgery | 8.1% | 4.2% | 0.005 |
| Target vessel vascularization | 16.5% | 14.5% | 0.4 |
| Major adverse cardiovascular events | 31.0% | 22.0% | 0.003 |