24

Occupational Lung Disease
General Description
Classification of Occupational Lung Disease
Occupational lung disease is an important medical problem: in 1986 the National Institute for Occupational Safety and Health (NIOSH) estimated that 28.5 million U.S. citizens worked in industries with a known risk for developing an occupational lung disease.1 Occupational lung diseases are caused by exposure to airborne organic and inorganic dusts, fumes, and gases in the workplace. Strictly speaking, the label “pneumoconiosis” should be reserved for those diseases caused by mineral dusts, but in practice it is often applied to any lung disease resulting from an occupational exposure. It is important to remember that some of the agents that produce occupational disease are also found in the home or the environment and may produce a clinically and pathologically identical lesion in these settings; common examples are hypersensitivity pneumonitis (see Chapter 21) and asthma (see Miscellaneous Conditions, later in the chapter, and Chapter 22).
This chapter discusses diseases caused by mineral dusts, fumes, gases, and some organic dusts. Hypersensitivity pneumonitis (extrinsic allergic alveolitis), a condition usually produced by organic dusts, is associated with exposures both within and outside the workplace and is discussed in Chapter 21. Malignant mesothelioma, although often caused by asbestos exposure, is covered in Chapter 26. More detailed descriptions of the pathologic reactions to inhaled occupational agents can be found elsewhere,2,3 as can extensive discussions of the clinical and radiographic features of occupational lung disease.4,5 We have also included in this chapter a brief section on the Effects of Particulate Air Pollutants and new or emerging occupational lung diseases (see Miscellaneous Conditions, later).6
Occupational diseases can be classified by disease or by etiologic agent; the latter is generally a simpler approach and is the approach used here. Thus one can broadly divide occupational lung diseases into those caused by gases and fumes, those caused by organic dusts, and those caused by mineral dusts. Further classification is then based on the specific diseases associated with each agent; for example, exposure to nitrogen dioxide causes both adult respiratory distress syndrome and bronchiolitis obliterans.
A further useful distinction is that between what might be termed classical pneumoconiosis caused by inorganic dusts, for example, silicosis, coal workers’ pneumoconiosis, and asbestosis, and hypersensitivity-type pneumoconiosis caused by either organic or inorganic dusts, for example, berylliosis, hard metal disease, and extrinsic allergic alveolitis (Table 24–1). In classical pneumoconioses, disease incidence is proportional to dose, and in general very high doses are required to produce disease. The latency period for most classical-type pneumoconioses is very long, typically years to decades, and this knowledge is useful in deciding whether a given pathologic process might represent a pneumoconiosis, or determining the exposures that might be responsible. Many classical pneumoconioses are characterized by fibrotic reactions of greater or lesser degree, and once fibrosis is established there is no current therapy that can reverse the process.
Classical Inorganic Dust–Type Pneumoconioses | Hypersensitivity-Type Pneumoconioses |
|---|---|
Disease incidence proportional to dose, and relatively high dose needed to produce disease | Often not clearly dose dependent, and low doses may cause disease |
Latency is long, typically decades | Latency variable and can be as short as a few weeks |
Fibrosis is the typical reaction |
|
No current therapy once fibrosis established | Demonstrable hypersensitivity reactions frequently present and may be useful diagnostic tools |
| Unusual pathologic reactions (often granulomatous) |
| Many cases respond to removal from exposure and steroid therapy |
Hypersensitivity-type pneumoconioses are quite different. Dose dependencies are often hard to show; the sensitizing dose can by very low, and the latency can be very short, in some instances only a few weeks. The presence of hypersensitivity can often be shown by skin testing or immunologic methods such as demonstrating specific serum precipitants in extrinsic allergic alveolitis, or lymphocyte blast transformation (beryllium lymphocyte proliferation testing) in berylliosis. Most important, hypersensitivity-type pneumoconioses often respond to cessation of exposure and steroids; hence early and accurate diagnosis can greatly improve the long-term outcome.
To avoid confusion and to ensure proper therapy, care should be taken to follow standard definitions in making diagnoses of occupational lung disease, because most of these etiologic agents can produce several distinctive conditions. Thus asbestos is known to cause asbestosis (diffuse interstitial fibrosis); this is a very specific clinical/morphologic entity. However, asbestos is a known cause of benign pleural disease and pleuropulmonary malignancies (see Asbestos-Related Disease, later), but these conditions are not, by definition, asbestosis. It is also important to bear in mind that occupational diseases are not just medical problems. Legal issues including reporting requirements and compensation may be involved, and may be greatly confused or compromised by the use of inappropriate terminology.
Clinical Features of Patients with Occupational Lung Disease
The interpretation of pulmonary pathology, more than many other fields of pathology, requires some familiarity with clinical aspects of lung disease including radiology and pulmonary function. In the present context, knowledge of a person’s occupations, present and past, and the potential for exposure to hazardous agents in such occupations, is crucial; a good history is by far the most important tool in the diagnosis of occupational lung disease.
The clinical features and pulmonary function changes in patients with occupational lung disease are in themselves nonspecific and all can be found in patients with nonoccupational lung disorders.7 The most common presenting symptom in the patient with occupational lung disease is shortness of breath.5,7 Cough and mucus hypersecretion can be seen in patients with industrial bronchitis, and the sputum may be distinctly colored as a result of the dust exposure. Wheezing may be present in the patient with occupational asthma. Fine rales over the lung bases are frequently heard in patients with diffuse interstitial diseases such as asbestosis or hypersensitivity pneumonitis. Patients exposed to high levels of toxic gases, fumes, and metal dusts, such as cadmium and beryllium, may present with adult respiratory distress syndrome (sometimes referred to in this setting as “chemical pneumonitis”) and respiratory failure. All long-standing fibrotic parenchymal lung diseases are associated with an increased risk of developing cor pulmonale.
Most of the conditions characterized by interstitial inflammatory infiltrates and interstitial fibrosis produce decreased diffusing capacity and a restrictive pattern of functional change. Complicated pneumoconiosis (progressive massive fibrosis) may show a very variable combination of obstructive and restrictive changes and is often associated with a reduced life expectancy. Although conditions that produce only macules or fine nodules (for example, simple coal workers’ pneumoconiosis and simple silicosis) may not be associated with functional abnormalities on routine spirometric testing, there is increasing evidence that some proportion of these patients have dust-induced dyspnea that may be detected on physiologic tests that measure small airway function8 or mismatches of ventilation to perfusion.9 The role of dusts in causing airway obstruction is discussed in several sections of this chapter.
Radiographic Features of Patients with Occupational Lung Disease
The radiographic appearances of the pneumoconioses are also in themselves nonspecific and may be seen in other types of lung disease. Furthermore, any given lung disease may manifest more than one pattern of abnormality. Despite these limitations, the chest film, in conjunction with a history of exposure, is probably the single most useful clinical tool for diagnosing pneumoconiosis.4,5
A standardized classification system for the pneumoconiosis has been developed by the International Labor Organization (ILO).10 Originally designed for evaluating nodular lesions such as silicosis, the current version permits the classification of asbestos-associated diseases as well as other pneumoconioses. The major applications of the ILO scheme are in ensuring comparability in epidemiologic research and in evaluating claims for compensation. To use this system, the radiologist, certified by NIOSH, compares the patient’s film with a set of standard anteroposterior (AP) chest films. By definition, the x-ray is assessed for technical quality, parenchymal abnormalities, and pleural and other changes.
Small Opacities | |
|---|---|
Major category | Subcategories |
0 (normal) | 0/− 0/0 0/1 |
1 (mild) | 1/0 1/1 1/2 |
2 (moderate) | 2/1 2/2 2/3 |
3 (severe) | 3/2 3/3 3/ |
Large Opacities | |
(Complicated Pneumoconiosis or Progressive Massive Fibrosis) | |
AX | Confluence of nodules >10 mm diameter |
Category A | Opacity with greatest diameter >10 mm but >50 mm |
Category B | One or more opacities larger than category A whose combined area does not exceed equivalent of right upper lobe (RUL) field |
Category C | One or more opacities whose combined area exceeds the equivalent of the RUL field |
If abnormalities consistent with pneumoconiosis are present, the profusion of small opacities are graded using the categories shown in Table 24–2. The small opacities are classified by shape into two subtypes, irregular and rounded; the former are typical of interstitial diseases such as asbestosis, whereas the former are seen in diseases with nodular lesions, for example, silicosis (Table 24–3). These are further subcategorized by size using the criteria shown in Table 24–3. Nodular lesions less than 1 cm in diameter are referred to as “simple” pneumoconioses and are graded into categories 1 to 3 with increasing profusion of opacities. Large opacities (greater than 1 cm in diameter) correspond to the lesions of “complicated” pneumoconiosis (otherwise known as “progressive massive fibrosis”) and are classified into categories, A, B, and C depending on their size (Table 24–2). Pleural and other changes are classified according to type (circumscribed or diffuse), location, width, extent, and presence of calcification. A summary of the more common radiographic features of the pneumoconioses are given in Table 24–4.
It should be appreciated that the categorization of a radiograph using the ILO scheme essentially functions to establish the presence/severity of a pneumoconiosis, but provides only a broad guideline to the cause of the pneumoconiosis. Thus arc-welders’ pneumoconiosis (siderosis), simple coal workers’ pneumoconiosis, and simple silicosis could produce identical chest roentgenographs. Determination of the exact disease requires historical exposure data or a pathologic specimen.
In recent years considerable effort has gone into using high-resolution computed tomography (CT) scanning to categorize pneumoconioses.11,12 There is considerable controversy about the use of CT scanning in this setting. Some authors believe that CT scans detect earlier disease than plain films, or that CT scans show less interobserver variation in interpretation,11,13 whereas others claim that CT scans are no more sensitive than plain films in detecting pneumoconiosis,12 and that many of the “early” findings detected by CT scans are actually nonspecific.14 At this point evaluation of posteroanterior (PA) films remains the standard for pneumoconioses.
Type | Size (mm) |
|---|---|
Rounded | |
p: diameter | 1.5 |
q: diameters | 1.5–3.0 |
r: diameters | 3.0 to approx. 10.0 |
Irregular | |
s: width | 1.5 |
t: width | 1.5–3.0 |
u: width | 3.0 to approx. 10.0 |
Pathologic Evaluation of Specimens from Patients with Occupational Lung Disease
The ability to assess the nature and severity of an occupational lung disease is directly proportional to the size of the pathologic specimen available for examination. Aside from the diagnosis of malignancies, transbronchial biopsies are of extremely limited utility in this field. Surgical (open and thoracoscopic) lung biopsies are usually considerably more informative, but, as indicated above, even this type of specimen requires careful correlation with clinical and radiographic findings.
In general, lung biopsies and whole lungs can be handled in much the same way as for nonoccupational lung disease. Formalin is probably the best fixative; it does not add heavy metals to the specimen and generally does not interfere with analytical procedures. It is critical that all open/thoracoscopic biopsy, resection, and autopsy specimens be fixed by inflation, as interpretation of any kind of disease process is made much more difficult in the presence of collapse. Churg15 described a simple method of inflating open lung biopsies with a needle (see Chapter 4). Lobes and lungs can be fixed by inflation via the bronchial tree (see Chapter 4).
Disease/Agent | Characteristic Roentgenographic Feature |
|---|---|
Coal workers’ pneumoconiosis | Small, rounded (sometimes combined with irregular) opacities predominate in upper lung fields; bilateral massive lesions (PMF) with advanced disease |
Silicosis | Alveolar filling pattern predominates |
Acute | Small, rounded opacities in upper lung fields; bilateral upper zone PMF with advanced disease; eggshell calcification of hilar lymph nodes |
Chronic |
|
Silicates | Nodular and/or irregular small opacities; may affect middle or lower ones predominantly; PMF may occur with advanced disease |
Rheumatoid pneumoconiosis | Rapidly enlarging opacities (1–5 cm) in all lung fields; mild or absent simple pneumoconiosis |
Asbestos | Small, irregular opacities predominately in lower zones; honeycombing in advanced disease |
Asbestosis |
|
Pleural disease | Circumscribed plaques and diffuse pleural thickening often present, with obliteration of costophrenic angles |
Relatively inert dusts of high radiodensity (iron, tin barium, antimony, zirconium, chromium, silver) | Very dense small, round opacities; may assume irregular appearance if exposure is high |
Relatively inert dusts of low radiodensity (e.g., limestone, cement, marble, gypsum) | Usually normal; abnormal film may indicate silica exposure |
Berylliosis |
|
Acute | Changes of adult respiratory distress syndrome |
Chronic | Nodular or linear opacities progressing to irregular nodularity and honeycombing; usually upper zonal |
Occupational asthma, bronchitis | Usually no change, but may show hyperinflated lungs |
Hypersensitivity pneumonitis | Reticulonodular opacities progressing to honeycombing |
Fumes |
|
Acute stage | Alveolar filling pattern (ARDS) |
Bronchiolitis obliterans | Finely nodular or reticular pattern |
Adapted from Churg A, Green FHY. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998, with permission.
Brightly Birefringent | Weakly Birefringent | Not Birefringent |
|---|---|---|
Silicate minerals (talc, mica, etc) | Crystalline silica | Asbestos, carbon |
Fillers in drugs intended for oral use (starch, microcrystalline cellulose) |
| Hard metal (tungsten carbide) |
Most specimens from patients with suspected occupational lung diseases do not require any special type of microscopic examination. Simple polarization may be used to provide an estimate of the amount of crystalline dust present (Table 24–5). This approach should be exercised with caution. Some dusts may be present in large amounts but are not birefringent in tissue sections; asbestos is an excellent example. Other dusts may polarize weakly and can be easily missed; this is true, for example, of crystalline silica. The color of visible dusts, and the presence of specific types of ferruginous bodies (see Asbestos-Related Disease, later) may also provide a valuable guide to the nature of the exposure. But no matter how much dust or how many ferruginous bodies are seen in a tissue section, the presence of dust (or bodies) indicates only exposure: dust does not, in and of itself, equal disease.
Formal quantitative analysis to determine the amount of a specific dust or mineral is only rarely necessary, but if such an analytical procedure is likely to be required (for example, digestion of the lung to determine the number of asbestos bodies present), it is preferable to retain a portion of the tissue specimen in formaldehyde rather than embedding it in paraffin. Preservation of fresh material by freezing is not required for establishing the diagnosis of an occupational lung disease, nor is it required for most microanalytical procedures.
A variety of analytical procedures are available for the identification of exogenous materials in lung samples, and the most appropriate analytical procedures for a given suspected exposure are suggested in the ensuing sections of this chapter. Further information is also available elsewhere.2,16 These procedures are an aid in establishing exposure by type and by quantity but are only indirectly relevant to the process of arriving at a diagnosis. To reiterate the point made above, exposure is not equivalent to disease, and no analytical procedure can substitute for a pathologic diagnosis.
Lastly, it is important to realize that pathologic and clinical findings may not always agree. Biopsy specimens tend to be more sensitive than pulmonary function tests or chest radiographs in detecting abnormalities, but whether such abnormalities are clinically important depends on the clinical setting. A biopsy showing features consistent with very mild asbestosis might be observed in a patient with a history of heavy asbestos exposure, a normal chest radiograph, and minimally altered pulmonary function tests, but if a biopsy shows what appears to be severe fibrosis in such a patient, then the biopsy almost certainly has sampled a nonspecific scar, and may actually provide no clue to the underlying disease. Similarly, it is not unusual, in our experience, to observe silicotic nodules in biopsies from patients with silicosis; however, it is rare for a patient to be biopsied to establish a diagnosis of silicosis. Rather, a biopsy is usually performed because a worker with known silicosis has some other radiographic process such as a mass suspected to be a carcinoma. In this setting a biopsy showing only a silicotic nodule has probably missed the lesion of clinical interest. Thus close collaboration with the pulmonary clinician is crucial to the accurate diagnosis of occupational lung disease.
Disease Caused by Inhaled Gases and Fumes
Exposure to gases and fumes (vapors produced by heating metals) is a common event, but one that is often overlooked as a cause of occupational lung disease. Gases and fumes produce a characteristic series of pathologic lesions (Table 24–6), but these lesions, for example, diffuse alveolar damage, are similar for all occupational agents and, obviously, are also common in patients without occupational exposure. Ascription of the pathologic findings to occupational exposure, therefore, depends to a great extent on a detailed exposure history and a knowledge of occupations with likely exposures to specific agents. Table 24–7 lists some specific gases and fumes and their likely occupational settings. Some metals may produce lesions both as fumes and as metal or metal oxide particles. Non–fume-related effects of metals, including lung cancer, are considered later (see Non–Fume-Related Lesions Caused by Metals and Related Compounds), as is occupational asthma (see Miscellaneous Conditions, later).
Adult respiratory distress syndrome (diffuse alveolar damage) |
Necrotizing bronchitis/bronchiolitis |
Bronchiolitis obliterans (constrictive bronchiolitis) |
Bronchiolitis obliterans with organizing pneumonia (cryptogenic organizing pneumonia) |
Bronchiectasis |
Industrial bronchitis |
Metal fume fever |
Occupational asthma |
Chronic airflow obstruction |
Agents | Uses/Occupations with Exposure |
|---|---|
Ammonia | Fertilizer manufacture |
| Pharmaceutical production |
| Plastic manufacture |
| Explosive manufacture |
Aluminum | Welding* |
Antimony tri- and pentachlorides | Coloring metals Chemical catalysts |
Beryllium | Welding (see text)* |
Cadmium | Soldering/welding cadmium alloys* |
Chlorine | Bleach manufacture |
| Chemical manufacture |
Chromium | Stainless steel welding* |
Copper | Welding* |
Fire smoke | Firemen |
Ammonia | Persons trapped in fires |
Acrolein |
|
Cyanide |
|
Chlorine |
|
Formaldehyde |
|
Nitric acid |
|
Phosgene |
|
Sulfuric acid |
|
Iron | Many types of welding* |
Hydrogen sulfide | Dye manufacture |
| Rubber manufacture |
| Sewer cleaners |
Manganese | Welding* |
Nickel | Stainless steel welding* |
Oxides of nitrogen | Farmers (silo work) |
| Explosive manufacture |
| Welding, electroplating |
| Metal cleaning |
| Diesel exhaust |
Ozone | Welding |
Phosgene | Welding |
| Fire fighting |
Sulfur dioxide | Chemical, pulp, and paper manufacture |
| Bleach and fumigation Smelters |
Zinc | Welding* |
*Most of these metals are also encountered in other settings, see particularly Non–Fume-Related Lesions Caused by Metals and Related Compounds and Table 24–14.
From Parkes WR. Occupational Lung Disorders, 2nd ed. London: Butterworths, 1982; Brooks SM. Lung disorders resulting from the inhalation of metals. Clin Chest Med 1981;2:235–254, Summer W, Haponik E. Inhalation of irritant gases. Clin Chest Med 1981;2:273–287, and Wright JL, Churg A. Diseases caused by gases and fumes. In: Churg A, Green FHY, eds. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998:57–76, with permission.
Adult Respiratory Distress Syndrome (Diffuse Alveolar Damage)
The association of adult respiratory distress syndrome (ARDS) with occupational inhalation exposures has only been clearly recognized within the past few decades,20,21 coincident with the clinical and pathological definition of ARDS in the nonoccupational setting (see Chapter 14). Prior to that time such cases were reported as “chemical pneumonia,” “chemical edema,” and “hemorrhagic edema,”22–24 and their pathologic features were not well described. It is now clear that the rapid onset of respiratory failure accompanied by widespread pulmonary infiltrates within hours after a gas or fume inhalation is exactly the same process seen in ARDS in the nonoccupational setting. Similarly, the recognition of the difference between true diffuse interstitial fibrosis, a process that takes months to years to develop, and organizing diffuse alveolar damage, a process that is well established within days or weeks, has made it clear that most of the cases reported in the older literature as “interstitial fibrosis” developing after an acute inhalation are really examples of organizing diffuse alveolar damage25–28 (see Chapter 14). Table 24–8 lists the reported occupational causes of ARDS, but this list is not exclusive, and most likely any irritant fume or gas inhaled in sufficient concentration will produce ARDS.
Agent | References |
|---|---|
Acetaldehyde | 17 |
Acrolein | 17 |
Ammonia | 29,30 |
Antimony tri- or pentachloride | 22 |
Beryllium | See Non–Fume-Related Lesions Caused by Metals and Related Compounds |
Boranes | 22 |
Cadmium and cadmium salts | 23,28,31–35 |
Chlorine | 22,36,37 |
Cobalt metal | 17 |
Fire smoke | 19,38 |
Hydrogen chloride | 17 |
Hydrogen sulfide | 22 |
Hydrogen selenide | 17,39 |
Mercury | 25,40 |
Methyl bromide | 41 |
Methylene chloride | 42 |
Nickel carbonyl | 43,44 |
Nitrogen dioxide | 45 |
Oil fly ash | 46 |
Ozone | 47,48 |
Perchloroethylene | 21 |
Phosgene | 22,42 |
Sulfur dioxide | 19,22,24,49 |
Titanium tetrachloride | 50,51 |
Zinc oxide and chloride | 26,27 |
Zirconium chloride | 22 |
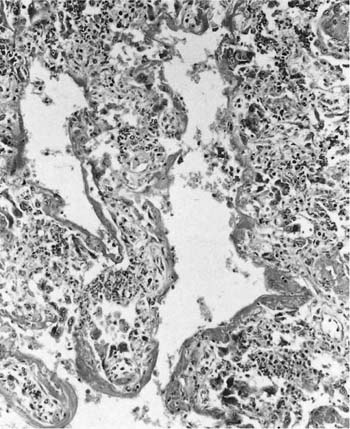
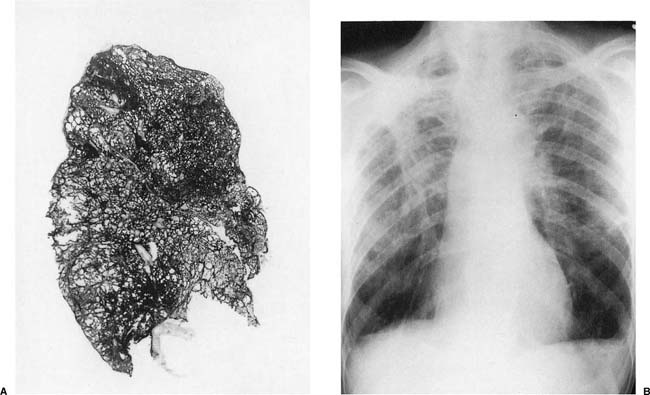
FIGURE 24–1 Microscopic appearance of diffuse alveolar damage (the pathologic equivalent of adult respiratory distress syndrome) showing typical hyaline membranes and collapsed alveolar parenchyma in a patient who inhaled perchloroethylene.
The pathologic features of diffuse alveolar damage caused by inhalation of gases and fumes are generally no different from those seen with other causes of diffuse alveolar damage (see Chapter 14) (Fig. 24–1). Although the acute forms with hyaline membranes and variable degrees of granulation tissue in airspaces are easily recognized, care should be taken not to mistake the organized forms with dilated respiratory bronchioles and alveolar ducts, and collapsed parenchyma with extensive granulation tissue for true interstitial fibrosis (see Chapter 14). Because of the morphologic nonspecificity of the lesions, a history of gas or fume exposure is required to indicate etiology.
Airway Damage Caused by Inhalation Exposures: Necrotizing Bronchitis/Bronchiolitis, Bronchiolitis Obliterans, and Bronchiectasis
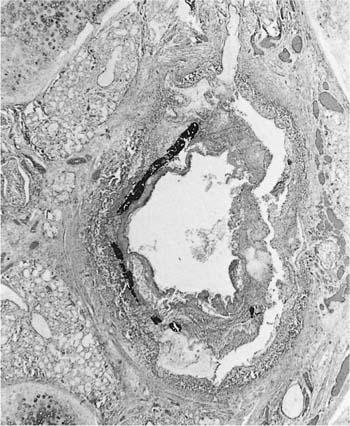
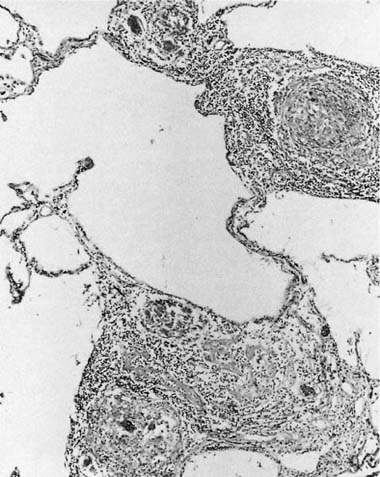
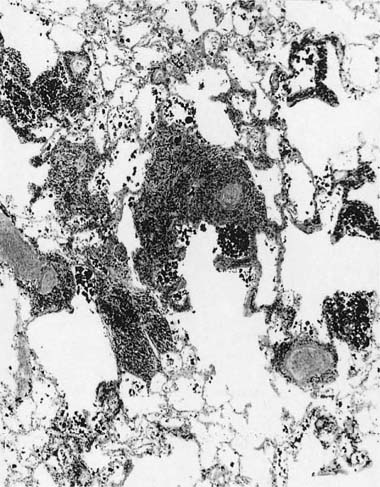
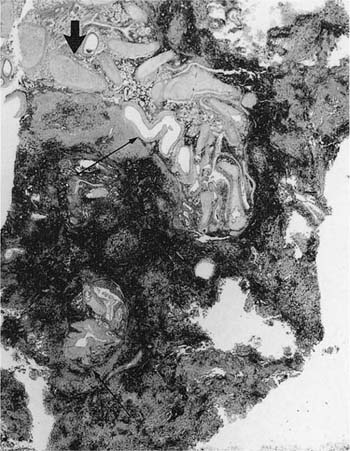
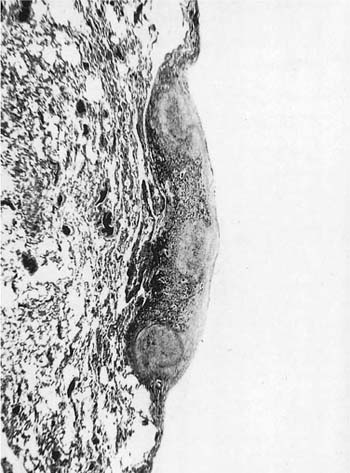
Many (if not all) of the agents that produce ARDS may also directly damage airway epithelium, and some also damage the airway wall.21 A variety of patterns are seen. Damage to the epithelium of the larger airways takes the form of a necrotizing bronchitis or bronchiolitis (Fig. 24–2). This is a relatively unusual lesion that should raise the suspicion of inhalation toxicity when it is encountered; however, similar lesions may be found with infectious processes, especially viral infections.
FIGURE 24–2 Micrograph showing bronchus with necrotic epithelium from a patient who was in a fire. The black material in the airway lumen is inhaled particulate matter from the smoke. This patient also developed adult respiratory distress syndrome.
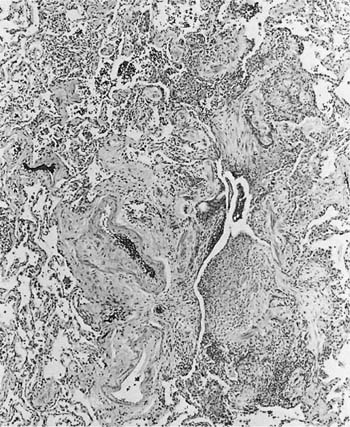
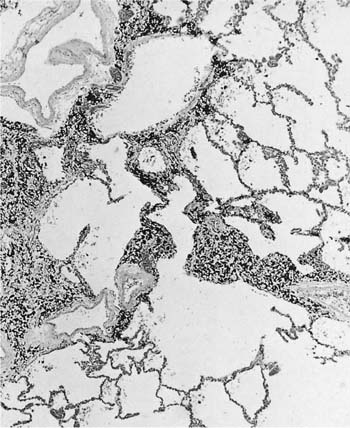
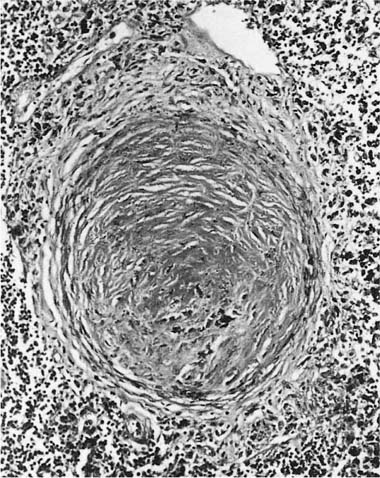
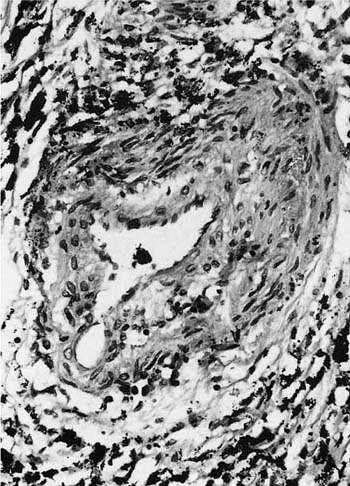
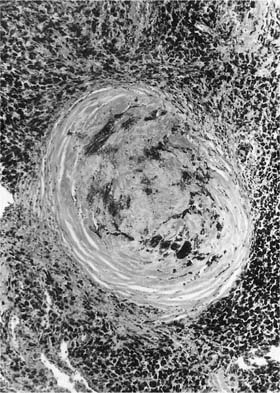
Damage to the epithelium of the membranous and respiratory bronchioles leads to production of the intraluminal tufts of granulation tissue classically referred to as bronchiolitis obliterans (Fig. 24–3). Reported occupational causes of bronchiolitis obliterans are listed in Table 24–9, but, again, it is likely that any irritant gas or fume can produce this lesion. A recent unusual observation was the finding of clinical bronchiolitis obliterans in workers at a plant making microwave popcorn;60 the offending agent was believed to be the ketone diacetyl, used in the artificial butter flavoring.
Why some exposures cause ARDS and some cause bronchiolitis obliterans is unclear. It has been proposed that small airway damage occurs with exposure to high concentrations of gases or fumes in enclosed spaces,56,57 and that highly soluble agents such as ammonia produce proximal airway damage whereas relatively insoluble agents such as nitrogen dioxide instead damage the distal airways;19,21 however, there is little concrete evidence supporting either of these proposals, and in some instances ARDS, necrotizing bronchitis, and bronchiolitis obliterans all occur in the same case. Reports of disease in workers who enter grain silos (“silo-filler’s disease”), and are exposed to nitrogen oxides that form on the top of silage, suggest that in fact exposures that are heavy but not immediately lethal cause ARDS, and lesser exposures (but perhaps more prolonged exposures because the lower gas concentration is less irritating) result in bronchiolitis obliterans.45
FIGURE 24–3 Bronchiolitis obliterans (proliferative bronchiolitis) in a patient who inhaled nitrogen oxide fumes in an industrial accident in a metal plating factory. Note masses of proliferating granulation tissue filling lumen of a respiratory bronchiole.
Ammonia |
Chlorine gas |
Diacetyl ketone* |
Nitrogen oxides |
Fire smoke |
Hydrogen selenide |
Sulfur dioxide |
*Based on clinical evidence.
From Sobonya R. Fatal anhydrous ammonia inhalation. Hum Pathol 1977;8:293–299, Schecter A, Shanske W, Stenszler A, Quintilian H, Steinberg H. Acute hydrogen selenide inhalation. Chest 1980;77:554–555, Woodford DM, Coutu RE, Gaensler EA. Obstructive lung disease from acute sulfur dioxide exposure. Respiration 1979;38:238–245, Murphy DMF, Fairman RP, Lapp NL, Morgan WKC. Severe airway disease due to inhalation of fumes from cleansing agents. Chest 1976;69:372–373, Seggev JS, Mason UG III, Worthen S, Staford RE, Fernandez E. Bronchiolitis obliterans. Chest 1983;83:169–174, Close LG, Catlin FI, Cohn AM. Acute and chronic effects of ammonia burns of the respiratory tract. Arch Otolaryngol 1980;106:151–158, Morgenroth K. Morphological alterations to the bronchial mucosa in high-dosage long-term exposure to sulfur dioxide. Respiration 1980;39:39–48, Arora NS, Aldrich TK. The use of steroids in bronchiolitis obliterans after smoke inhalation. Crit Care Med 1981;9:72–73, Darke CS, Warrack AJN. Bronchiolitis from nitrous fumes. Thorax 1958;13:327–333, Ploysongsang Y, Beach BC, DeLisio RE. Pulmonary function changes after acute inhalation of chlorine gas. South Med J 1982;75:23–26, Woodford DM, Coutu RE, Gaensler EA. Obstructive lung disease from acute sulfur dioxide exposure. Respiration 1979;38:238–245, Kreiss K, Gomaa A, Kullman G, Fedan K, Semoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave popcorn plant. N Engl J Med 2002;47:330–338, and Perez-Guerra F, Walsh RE, Sagel SS. Bronchiolitis obliterans and tracheal stenosis: Late complications of inhalation burn. JAMA 1971;218:1568–1570, with permission.
The clinical picture of bronchiolitis obliterans is usually considerably different from that of ARDS. Patients who develop bronchiolitis obliterans typically report an acute inhalation exposure that may produce cough and considerable chest discomfort, but then apparently resolves, only to be manifested several weeks later as progressive shortness of breath. Such patients are found to have variable, but sometimes quite severe, airflow obstruction, and may demonstrate a radiographic appearance of hyperinflation.19,38,39,45
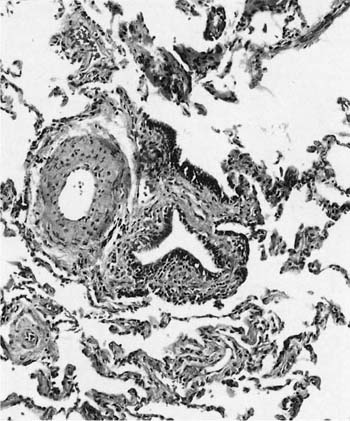
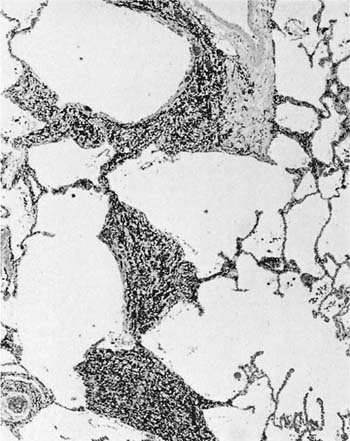
The histologic classification of bronchiolitis obliterans of noninhalation origin has been a matter of controversy. Colby and Carrington63 have proposed that such cases be categorized as either proliferative bronchiolitis (Fig. 24–3), in which the airway lumen contains granulation tissue, or constrictive bronchiolitis, in which the granulation tissue has scarred down to produce an irregularly narrowed lumen. The same classification can be applied to bronchiolitis obliterans of inhalational origin; although the proliferative lesions are readily recognized, the more subtle constrictive lesions are easily missed (Fig. 24–4). This histologic distinction is of considerable clinical importance, because the proliferative lesions may resolve, whereas cases characterized by widespread constrictive bronchiolitis usually have irreversible airflow obstruction (see Chapter 21). Some cases of proliferative bronchiolitis of inhalational origin appear to respond to steroids.19,56,64
Bronchiectasis has been reported after exposure to corrosive substances, more commonly as liquids than as gases30,31,54,61,62 (Table 24–10); the usual setting is that of an industrial accident, or of accidental aspiration in childhood (Fig. 24–5). Presumably this process reflects either direct damage to the walls of large airways by the inhaled agent or an extremely intense inflammatory response to the agent with subsequent weakening of the wall and dilatation.
Ammonia |
Fire smoke |
Kerosene |
Phosgene |
Nickel carbonyl |
From Sobonya R. Fatal anhydrous ammonia inhalation. Hum Pathol 1977;8:293–299, Weston JT, Liebow AA, Dixon MG, Rich TH. Untoward effects of exogenous inhalants on the lung. J Forensic Sci 1972;17:199–279, Close LG, Catlin FI, Cohn AM. Acute and chronic effects of ammonia burns of the respiratory tract. Arch Otolaryngol 1980;106:151–158, Hoeffler HB, Schweppe HI, Greenberg SD. Bronchiectasis following pulmonary ammonia burn. Arch Pathol Lab Med 1982;106:686–687, and Perez-Guerra F, Walsh RE, Sagel SS. Bronchiolitis obliterans and tracheal stenosis: Late complications of inhalation burn. JAMA 1971;218:1568–1570, with permission.
FIGURE 24–4 Bronchiolitis obliterans (constrictive bronchiolitis) in an arc welder. The airway wall is distorted by fibrous tissue and the lumen irregularly narrowed.
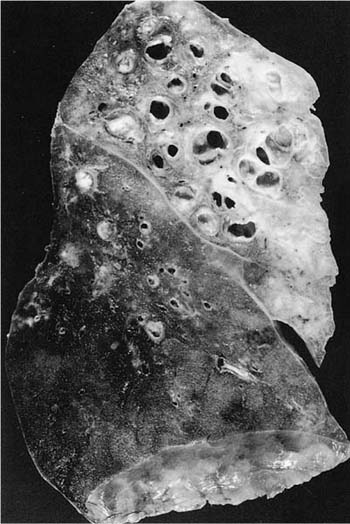
FIGURE 24–5 Severe upper lobe bronchiectasis in a patient who inhaled kerosene as a child and lived to adulthood.
Fumes | Gases |
Cadmium | Ozone |
Nickel | Nitrogen oxides |
Chromium | Phosgene |
Iron | Phosphine |
Beryllium |
|
Manganese |
|
From Sferlazza SJ, Beckett WS. The respiratory health of welders. Am Rev Respir Dis 1991;143:1134–1148, with permission.
Effects of Welding
Welding denotes any process of joining metals by heat or pressure. A detailed description of the effects of welding is beyond the scope of this chapter, but the interested reader is referred elsewhere65,66 for a review. Welding is a fairly common occupation (some 400,024 workers are estimated to be exposed to welding fumes or work in related occupations66), and one that leads to a complicated set of gas, fume, and particle inhalations. Depending on the type of welding process, the electrodes being used, and the metals being joined, welders may be exposed to a variety of hazardous fumes and gases (Table 24–11); because of the multiplicity of potential exposures, sorting out the effects of welding has been difficult.
Table 24–12 lists diseases known or proposed to be caused by exposure to welding fumes. ARDS is apparently often fairly mild65 (and referred to as “chemical pneumonitis”), and has been particularly associated with ozone and cadmium exposure;33,48,67 the latter has occasionally been fatal.34,35 Hypersensitivity pneumonitis is said to be difficult to distinguish from mild forms of ARDS after welding fume exposure, but is typically associated with fever and rigors and multiple occurrences that increase in severity with each exposure.47 Bronchiolitis obliterans (constrictive bronchiolitis) appears to be poorly documented as a pathologic entity occurring in welders, but we have seen a few cases in which welding exposures were the most likely causes of the airway lesions20 (Fig. 24–4).
Metal fume fever is characterized by a syndrome of excessive thirst and a metallic taste in the mouth 4 to 8 hours after exposure to metal fumes, followed by the development of fevers, myalgias, rigors, and weakness, and peripheral leukocytosis. Symptoms subside after 24 to 48 hours, and the chest roentgenograph usually remains normal despite the presence of crepitations on auscultation. The disease is thought to be a reaction to fumes and fine metal particles, but, as opposed to occupational asthma and hypersensitivity pneumonitis, there is no latent period for sensitization, and repeated exposures induce tolerance. However, tolerance is rapidly lost, so that symptoms typically recur on Mondays after a weekend away from work and then improve over the course of the workweek. Bronchoalveolar lavage 24 hours after exposure typically shows increased numbers of polymorphonuclear leukocytes.
Metal fume fever is most commonly reported in workers exposed to zinc fumes, but disease with exposures to copper, magnesium, chromium, manganese, nickel, titanium, and cadmium fumes has been described.68 A similar syndrome has been reported after exposure to polytetrafluorethylene fumes in the manufacture of Teflon (“polymer fume fever”).65
There is considerable uncertainty about the effects of welding exposures in producing chronic airflow obstruction. Industrial bronchitis, which may be seen in welders, refers to chronic cough and mucus production caused by exposure to a particle, fume, or gas;69 it is the occupational equivalent of cigarette-smoke–induced chronic bronchitis, and similarly produces little if any functional impairment. Evaluation of the published data suggests that welding in general does not lead to severe or clinically apparent airflow limitation, although there is some evidence that the combination of welding fume and cigarette smoke is deleterious. Functional abnormalities that have been found have been mild and suggestive of small airway disease.65 However, most of the data have been derived from cross-sectional studies, and longitudinal studies are necessary to definitively rule out long-term effects. Flow limitation, if it occurs, may be related to the presence of iron-laden dust macules (visible as a radiographic pneumoconiosis, usually termed “siderosis” in welders) and subsequent small airway fibrosis; this is discussed later (see Non–Fume-Related Lesions Caused by Metals and Related Compounds). A number of cases of true occupational asthma with marked flow limitation have been reported in welders, more often as a result of exposure to organic agents in the welding fluxes than to the metal fumes themselves70,71 (see Byssinosis and Occupational Asthma, in Miscellaneous Conditions, later).
Adult respiratory distress syndrome |
Metal fume fever |
Hypersensitivity pneumonitis |
Occupational asthma |
Industrial bronchitis |
Pneumoconiosis (siderosis) |
? Bronchiolitis obliterans |
? Chronic airflow obstruction |
? Lung cancer |
? Diffuse interstitial fibrosis |
From Sferlazza SJ, Beckett WS. The respiratory health of welders. Am Rev Respir Dis 1991;143:1134–1148; and Antonini JM, Lewis AB, Roberts JR, Whaley DA. Pulmonary effects of welding fumes. Am J Ind Med 2003;43:350–360, with permission.
Most welders who develop radiograph changes have small nodules (macules) (see Non–Fume-Related Lesions Caused by Metals and Related Compounds, later). However, there are a few cases of diffuse interstitial fibrosis reported in welders.66 In some instances fibrosis may actually be related to the effects of coexposures, particularly asbestos, because many welders in the past worked in shipyards and had high levels of asbestos exposure. However, we have seen rare cases in which diffuse interstitial fibrosis was accompanied by large amounts of iron visible in the fibrotic areas and in which there was no evidence of significant asbestos exposure; these cases may represent diffuse fibrosis induced by retention of very large amounts of welding fume.
Some studies have suggested that welders, particularly those welding chromium-containing stainless steel, may be at increased risk of lung cancer, but this question is disputed and the published data are inconsistent.65,66,72–76 Some reports suggest that asbestos exposure or high rates of cigarette smoking may account for much of the claimed increases in lung cancer risk.74–76
Non–Fume-Related Lesions Caused by Metals and Related Compounds
Metals and related compounds (metal salts, metal oxides) produce a variety of lesions related to particle rather than fume inhalation (Table 24–13).68 However, there is considerable variation in the frequency with which specific lesions are found; dust macules, for example, are seen with many different dusts, whereas relatively few dusts produce interstitial fibrosis, and many of the reports of interstitial fibrosis are of questionable validity20 (see later). Some dusts such as hard metal and beryllium operate through immunologic mechanisms and produce fairly distinctive lesions.
Table 24–14 lists the uses and sources of exposure for different metals and related compounds, and the reported lesions. Dust macules, small airway lesions, and emphysema are described here as general topics, as are interstitial fibrosis and occupational lung cancers. More details are then provided in the sections on specific dusts.
Dust macules |
Small airway disease |
Interstitial fibrosis |
Lung cancer |
Alveolar proteinosis |
? Emphysema |
Giant cell interstitial pneumonia (hard metal disease) |
Granulomas (beryllium) |
Dust Macules, Small Airway Lesions, Emphysema, and Airflow Obstruction in Workers Exposed to Metals and Related Compounds
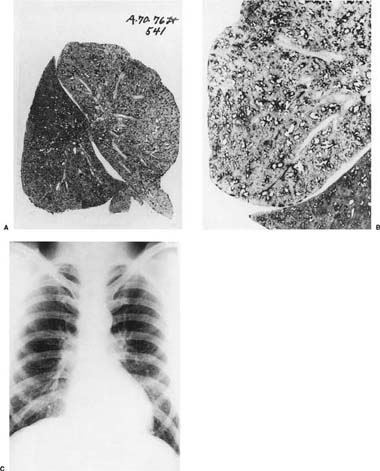
A dust macule is defined as a nonpalpable nonfibrotic pigmented lesion that microscopically consists of dust, either free or in macrophages, around small airways and vessels17,20 (Fig. 24–6). Many metals and related compounds produce dust macules and most produce no other lesions. When macules are found pathologically, or nodules (see below) are found roentgenographically, specific disease names can be used; for example, in those with iron exposure, the process is called siderosis, and with tin exposure, stannosis.
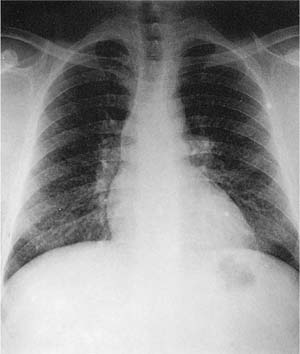
Because the dusts making up the lesions are radio-dense, macules appear as fine nodules (simple pneumoconiosis, as defined earlier in the chapter), generally more prominent in the upper zones, on chest roentgenographs (Fig. 24–7). With very radiodense metals such as tin or barium, the chest roentgenographs may be spectacular (an example is provided elsewhere17, pp. 118–122). Such roentgenographs may also show Kerley B lines, reflecting collections of dust along interlobular septa.
Grossly, dust macules appear as colored “spots” in the centers of the lobules; similar (unnamed) collections of dust frequently extend along the interlobular septa and pleural lymphatics. In addition to metals, many other types of dust (coal, silica, silicates) also produce macules. The exact color depends on the nature of the dust; the most common macule is the collection of black pigment seen in the centers of lobules in cigarette smokers and many city dwellers, the latter from inhaled particulate air pollutants. Some dusts typically produce specific colors; for example, the red-brown lesions found in hematite miners, the black macules seen in coal miners (see Coal Workers’ Pneumoconiosis and Related Diseases, later), the gray lesions caused by tin, and the white macules in those exposed to the paint pigment titanium oxide.
The macule was originally defined to distinguish these lesions, which were thought to be nonfibrotic “blemishes” of little functional impact, from the heavily collagenized nodules of silicosis,17,20 a form of pneumoconiosis thought to produce major functional impairment (this in fact is not true for most cases of simple silicosis; see Silicosis and Other Diseases Caused by Silica, later). From this separation arose the concept that dusts producing only macules were “inert.” However, it has become clear that, although macules may initially have little associated fibrosis, with time or continued dust accumulation, considerable fibrosis may occur, and that, with sufficient exposure, no dust is truly inert.
Agents | Diseases | Occupations/Processes with Exposure |
|---|---|---|
Aluminum | Interstitial fibrosis | Mining bauxite |
| Alveolar proteinosis | Aluminum smelting |
| ?Lung cancer (pot room workers) | Production of aluminum metal or specific products |
|
| Aluminum grinding, polishing, welding |
Antimony | Macules | Mining, processing, and smelting antimony ores |
|
| Production/use of antimony alloys |
|
| In paints, glass |
|
| Linotype setting |
Barium | Macules (Baritosis) | Mining/refining ores |
|
| Glass manufacture |
|
| Filler for paints, papers, textiles |
|
| “Sand” blasting |
|
| Medical contrast agent |
Beryllium | Granulomas | See Table 24–16 |
| Nodular fibrosis |
|
| Diffuse interstitial fibrosis |
|
| ARDS |
|
Cadmium | ARDS | High friction metal alloys |
| ?Emphysema | Electroplating |
| ?Lung cancer | Paints, ceramics, glass, plastics |
|
| During refining of other ores (zinc, lead, copper) |
Chromium | Lung cancer | Mining/refining |
|
| Chrome plating |
|
| Paints and pigments (as chromates) |
Cobalt/hard metal | Interstitial fibrosis | Hard metal production/use |
| “Hard metal disease” | In alloys and steel |
| Hypersensitivity pneumonia | As paint and china pigment |
| Contact dermatitis | Catalyst in the oil and chemical industry |
| Occupational asthma |
|
Copper | Interstitial fibrosis (rare) | Mining/refining ores (also produces exposure to silica, arsenic,cadmium) |
Iron | Macules (siderosis) | Iron and steel mill work |
| ?Interstitial fibrosis | Steel grinding/cleaning |
| Nodules (? with silica) | Foundry work |
| Boiler scaling |
|
| Mining hematite, magnetite, limonite,siderite |
|
| Mining or processing emery |
|
| Mining iron containing clays |
|
Lanthanum/rareearths | Macules | Mining/refining ores (also produces exposure to silica) |
| ?Interstitial fibrosis | Polishing glass (cerium) |
|
| Glass manufacture |
|
| High temperature ceramics and alloys |
|
| In carbon arc lamps |
Nickel | Lung cancer | Mining |
|
| Welding |
|
| Stainless steel fabrication |
Silicon carbide(carborundum) | Interstitial fibrosis | Manufacture of silicon carbide abrasives |
| ?Lung cancer |
|
Tin | Macules (stannosis) | Tin mining (also produces exposure to silica) |
|
| Refining of tin ore |
|
| Tinplating/soldering |
|
| In fungicides/pesticides |
Titanium | Macules | Manufacture of ceramics, paints |
|
| Machining titanium metal |
Zirconium | Macules | Mining/refining ores (also produces exposure to silica) |
|
| Production of steel |
|
| Manufacture of refractory ceramics |
|
| In foundries as sand substitute |
|
| In enamels and glasses |
|
| As a polishing and abrasive agent |
Modified from Wright JL, Churg A. Diseases caused by gases and fumes. In: Churg A, Green FHY, eds. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998:57–76, with permission.
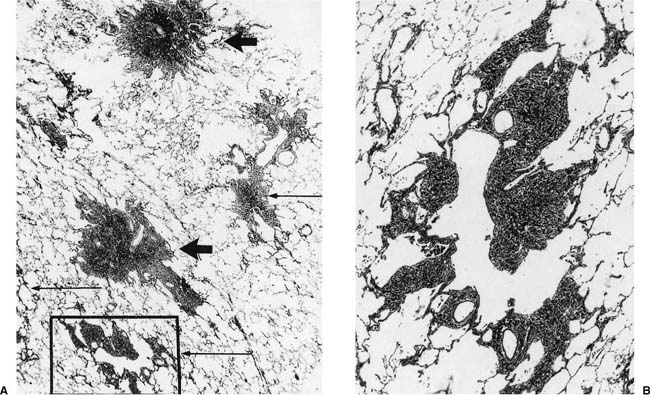
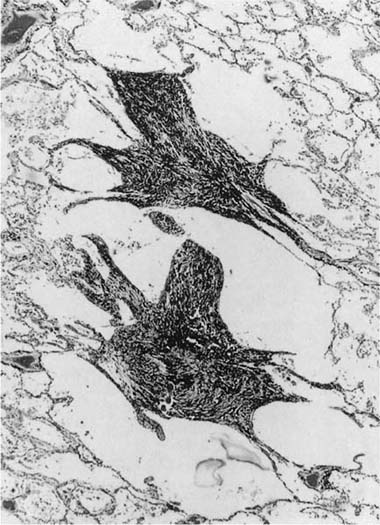
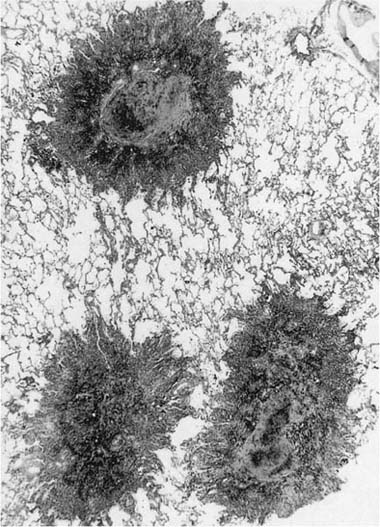
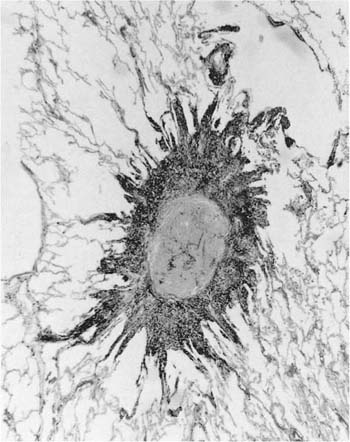
FIGURE 24–6 So-called macule in the lung of a hematite miner. The lesion consists of pigment and fibrous tissue in the walls of respiratory bronchioles. This lesion, which demonstrates some distortion of the airway, is identical to the pathologic change that we have designated “mineral dust–induced airways disease” (see text). Compare to Figs. 24–13B and 24–41. (Case courtesy Dr. A. Katzenstein.)
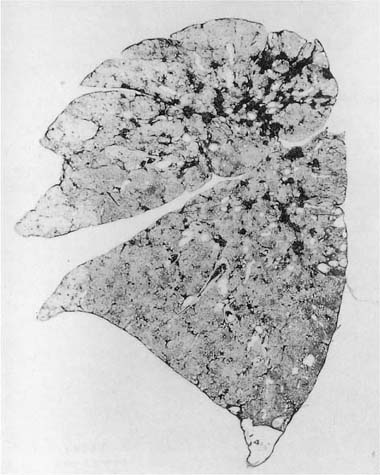
FIGURE 24–7 Chest radiograph showing finely nodular infiltrate in the lungs of a welder. This appearance is nonspecific and might be seen in a coal miner with coal workers’ pneumoconiosis, or in a worker with silicosis. (From Churg and Green,2 with permission.)
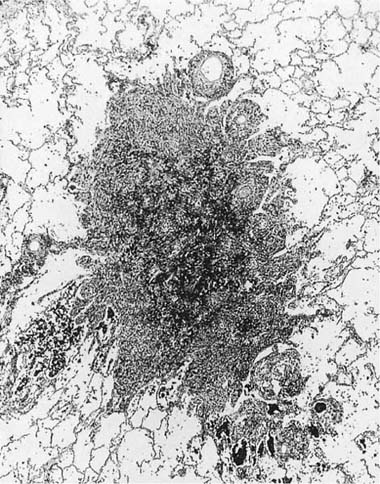
We have described elsewhere fibrotic lesions of the small airways caused by mineral dusts,77,120–123 and proposed that these be called “mineral dust–induced small airways disease” or “particle-induced small airways disease.”77,124 These lesions appear to represent progression of the true nonfibrotic macule to airway scarring. In its simplest form the lesion consists of linear accumulations of collagen and pigment that extend down the small airways (membranous and respiratory bronchioles) and even sometimes into alveolar ducts. With progression the airway walls gradually become quite thickened and the airway distorted77,124 (Fig. 24–6), and in its most marked form the lesion consists of a central collagenized scar frequently associated with surrounding enlarged airspaces in which the relationship to an underlying airway is difficult to discern (Fig. 24–8).
The terminology here is quite confusing because for some specific pneumoconioses, such as coal workers’ pneumoconiosis, the distorted and fibrotic airway is still explicitly referred to as a “macule” and the surrounding enlarged airspaces as “focal emphysema” (see Coal Workers’ Pneumoconiosis and Related Diseases, later). Whether focal emphysema is really a separate process from airway fibrosis and distortion is unclear. But whatever terminology one applies, it appears that these morphologic abnormalities are in fact common stereotypic reactions to a wide variety of mineral dusts including metals such as iron and aluminum, coal, asbestos, and many nonasbestos silicates.77,120–124 Even particulate air pollution particles can produce similar changes with long-standing high level exposure (see Effects of Particulate Air Pollutants, later).
The pathogenesis of these lesions has been recently reviewed.124 Airway wall fibrosis appears to reflect high dust loads derived either from direct deposition of particles in the small airways or proximal drainage of particles phagocytized by macrophages, and the subsequent long-standing inflammatory response that these dusts evoke within the airway wall. As well, dusts that enter the airway wall may in themselves evoke fibrotic reactions, largely through oxidant-driven mechanisms.125 The pathogenesis of focal emphysema is less clear. However, the prolonged inflammatory response caused by dust deposited in the airspaces may lead to tissue destruction because of chronic neutrophil/macrophage recruitment and excess local proteolysis in much the same fashion as is believed to be true of cigarette smoke (see Chapter 22).
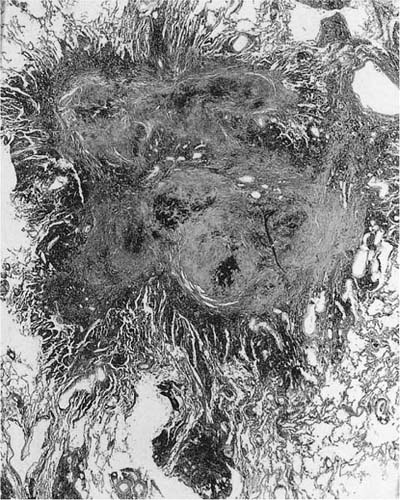
FIGURE 24–8 So-called macule with surrounding enlarged airspaces in the lung of the same hematite miner illustrated in Fig. 24–6. This lesion appears morphologically identical to the combination of airway fibrosis (macule) and focal emphysema seen in coal workers’ pneumoconiosis (compare Fig. 24–14). (Case courtesy Dr. A. Katzenstein.)
The recognition that mineral dusts produce small airway fibrosis and a form of centrilobular emphysema has occurred at the same time as mineral dust exposure has become accepted as a cause of chronic airflow obstruction (reviewed elsewhere126–131). Again, the association was first recognized in coal workers, but has been extended to silica exposure126–133 and to asbestos exposure.126–131,134–137 The issue of exactly what lesion is responsible for airflow obstruction is unresolved, and, although (focal) emphysema126–128,132,133 is often proposed as the underlying abnormality in workers with coal and silica exposure, both human and experimental data imply that airway fibrosis may be equally or even more important.77,125
The exact frequency and clinical significance of dust-induced airflow obstruction require further elucidation. Longitudinal studies based on groups of workers suggest that the effects of high-level dust exposure on functional decline can be as great as the effects of cigarette smoke, and the two agents may in fact be synergistic126–131 (see also Coal Workers’ Pneumoconiosis and Related Diseases, later). Although accelerated rates of functional loss do not in themselves necessarily indicate the presence of clinical disease, the implication of the longitudinal epidemiologic surveys is that a minority of workers will in fact develop significant airflow obstruction because of dust exposure.130,138
The possible association of cadmium exposure and emphysema is discussed in the subsection on cadmium, later.
Interstitial Fibrosis
Interstitial fibrosis is a relatively rare finding in workers exposed to metals and related compounds, and it is unclear in many instances whether dust exposure is really the cause of the disease; for example, the few cases of diffuse interstitial fibrosis seen in workers exposed to vapors from rare-earth–containing arc lamps.139,140 Similarly, the nature and exact cause of the nodular/diffuse fibrotic lesions seen in workers who spray vineyards with mixtures of copper sulfate and lime as a fungicide is unclear.141,142 Lastly, so-called dental technician’s pneumoconiosis is a condition seen in workers who make dental prostheses. The prostheses contain or are made using a variety of substances including chromium, cobalt, molybdenum, beryllium, and nickel, and they may be sandblasted with silica sand.143 Chest roentgenographs have been reported as showing reticulonodular infiltrates; the few pathologic specimens have been claimed to resemble berylliosis, silicosis, and hard metal disease.144–147
Exposures to beryllium, aluminum, cobalt/hard metal, and silicon carbide have all been associated with diffuse interstitial fibrosis. These specific agents are considered later.
Lung Cancer
Occupational exposure to a number of metals, metal salts, gases, silica, and silicate minerals (asbestos) has been accepted or proposed to be associated with the development of lung cancer (reviewed elsewhere83,148) (Table 24–15), but even some of the “accepted” causes (meaning that they have been classified as human carcinogens by the International Agency for Research on Cancer) are controversial; examples are crystalline silica and beryllium.87,107,111 Asbestos as a carcinogen is discussed at length in Asbestos-Related Disease, later, and will not be considered here, and the possible relationship of silica exposure and lung cancer is presented in Silicosis and Other Diseases Caused by Silica, later. It should be appreciated that these associations are based largely on epidemiologic studies, and sometimes on experimental animal models, and that in some instances identifying the exact causative agent may be difficult. Sometimes the carcinogenic agent is the substance being handled or refined, for example, nickel or chromate salts, but in other instances the carcinogen is a contaminant or another substance related to the industrial process. Thus smelting of copper, zinc, and aluminum has been associated with an increased cancer risk,78–94 and this is believed to be caused by arsenic in the ores being smelted. Underground mining of many substances that in themselves are clearly not carcinogens, for example, iron ore, is associated with an increased lung cancer risk because of the presence of radon in the mines.116–118,149 As well, many types of metal particles, which in themselves fail to induce cancers in animals, do increase the experimental yield of tumors when administered with known carcinogens such as benzo[a]pyrene, possibly by increasing transport of carcinogens into the respiratory epithelium, or by altering the metabolism of carcinogens.150,151 A further complicating factor is that many industrial settings combine exposure to mineral dusts with exposure to numerous other substances, some of which are known or potential carcinogens; this is particularly true for foundry workers and aluminum pot room workers110 and welders.72,73 Lastly, other pathologic processes in the lung, particularly fibrosis, caused by dust exposure may also be important in carcinogenesis. For example, there is strong evidence that lung cancer in asbestos workers is linked to the presence of asbestosis (diffuse interstitial fibrosis) rather than to asbestos alone (see Asbestos-Related Disease, later); similarly, the strongest evidence for an association of silica exposure and lung cancer is seen in those with silicosis (see Silicosis and Other Diseases Caused by Silica, later).
Agent | References |
|---|---|
Accepted/probable carcinogens/exposures |
|
Asbestos | See text (Asbestos-Related Disease) |
Arsenic | 78–83 |
Beryllium | 83–88,111 |
Chromates | 83,89,90 |
Chloromethyl ether | 91–95 |
Nickel | 83,89,96,97 |
Radon and radon daughters | 98–105 |
Silica | 106–109 |
Smelting (arsenic exposure) | 78–82,110 |
Possible carcinogens/exposures |
|
Aluminum pot room work | 110 |
Cadmium | 83,112,113 |
Foundry work | 114,115 |
Iron mining | 116–118 |
Silicon carbide manufacture | 119 |
Welding | 66,73–76 |
Showing an association between a given agent and the development of lung cancer remains primarily an epidemiologic task, and any attempt to ascribe a given exposure to the development of a lung cancer requires supporting epidemiologic evidence. The important question for the pathologist is whether there are pathologic features that help make this association in a given case. Tumor location (lower zone) has been proposed as indicating an asbestos etiology (see Asbestos-Related Disease, later), but is of doubtful use with asbestos exposure and clearly no use for other exposures. Tumor cell type has also been suggested as a specific marker in some circumstances, but careful review indicates that most occupational lung carcinogens produce exactly the same spectrum of histologic types as does cigarette smoking.152,153 The only possible exceptions are the high incidences of small cell carcinoma seen in workers with radiation exposure (particularly uranium miners) and workers exposed to chloromethyl ether.91–95,98–105 Given the rarity of small cell carcinoma in nonsmokers in the general population, in these particular occupational settings a small cell carcinoma arising in a nonsmoker might reasonably be ascribed to occupational exposure, but in smokers there is no obvious way to decide that occupational exposure rather than smoking was the causative agent.
Specific Agents
Iron and Other Inert Dusts
Iron is probably the metal most commonly encountered in the occupational setting with as many as 4,024,024 workers in the United States exposed.18 The disease caused by inhalation of iron alone is referred to as “siderosis,” and when free silica is also present, as “silicosiderosis,” a form of mixed dust (i.e., silica plus another dust) fibrosis. Iron exposure is seen in those mining hematite, magnetite, limonite, and siderite, all different iron ores, as well as in those mining and milling emery (aluminum oxide), which is used for grinding, and crocus, a finely divided form of iron oxide used for polishing.17,18 Iron-containing ores are also used in the manufacture of paint pigments. Extensive iron exposure is common in steel mills, foundries, welding, silver and steel grinding and polishing, boiler scaling, and production and refining of many metal alloys. These occupations may also entail considerable silica exposure; this is particularly true of mining and of foundry work. As noted previously, iron exposure, along with numerous other exposures, is encountered in welding.
Iron is considered the prototypical “inert” dust, and exposure to iron in and of itself is generally believed to produce roentgenographic abnormalities, but no clinical disease;17,154,155 that is, siderosis is regarded as a “benign” pneumoconiosis. However, iron exposure does produce macules and fibrotic airway lesions,77 and it is possible that iron-exposed workers develop airflow obstruction, as discussed previously. The chest roentgenograph in iron-exposed workers frequently shows upper zonal fine nodules; in a study of 661 welders, Attfield and Ross156 found a 7% prevalence of small rounded opacities. Total retained iron burden correlates with years of exposure and with the extent and severity of roentgenographic changes.157,158 The generally low toxicity of iron can be appreciated from the enormous burdens, occasionally as high as 10 g,159 that may be found in the lungs. After cessation of exposure, iron is cleared slowly from the lung and roentgenographic changes may regress,158 in contradistinction to dusts such as silica and coal.
Macroscopic examination of lungs from iron-exposed workers usually reveals macules that vary from red to brown, gray, or black,160 depending on the exact exposure. Microscopically, the dust appears brown to black, both free in the lung and aggregated in macules and fibrotic small airway lesions (Fig. 24–6). Many of the particles appear as rounded ferruginous bodies with round or polygonal black cores (see Asbestos-Related Disease, later). Exogenous iron does not stain with Prussian blue, but many of the particles in lungs from iron-exposed workers do stain, most likely because the exogenous particles have become ferruginated. Dense nodular fibrosis is also sometimes seen and has been attributed to concomitant exposure to silica,160–164 but some experimental studies suggest that very large amounts of iron or welding fume may be able to produce nodular fibrosis as well.165,166 This issue remains unresolved.
As noted in the description of the effects of welding, we have seen a few examples of individuals in whom high, microscopically visible, iron loads appear to be associated with interstitial fibrosis, and occasional cases with a restrictive pattern of pulmonary function after iron exposure have been published.68
So-called benign pneumoconioses are also produced by exposure to tin, barium, antimony, zirconium, chromium ore, and titanium. Roentgenographically all are characterized by very dense nodules and sometimes Kerley B lines as well.18 The commercial uses of these substances are listed in Table 24–14. The number of exposed workers is greater than might be supposed: NIOSH has estimated that 1,400,024 workers in the United States are exposed to antimony, and 1,300,024 to zirconium.18 Pathologic descriptions are few, but suggest that these agents generally produce macules and less commonly airway fibrosis.167–172 Barium salts, tin oxide, and titanium oxide can be detected by polarization of tissue section, and barium sulfate often has a greenish tinge in hematoxylin and eosin (H&E)-stained sections.
Aluminum
Aluminum has numerous uses (Table 24–14). NIOSH estimated that almost 4,024,024 workers in the United States are exposed to alumina (Al2O3) or various forms of aluminum metal.18 Occupational exposures may be complex: alumina is extracted from bauxite ore (alumina, iron, and other minerals) and then reduced to metallic aluminum by electrolysis using large pots (in the “pot room”) holding up to 10 tons of mixture.17,18,173 The pot room atmosphere contains not only alumina dust, but also a variety of other substances including hydrogen fluoride, sulfur dioxide, metal fumes, cyanide, ozone, and aromatic hydrocarbons.17,18,173
Exposure to aluminum, alumina, and pot room fumes has been reported to produce a variety of pathologic reactions including chronic airflow obstruction, asthma, and interstitial fibrosis; almost all are a matter of dispute. Diffuse interstitial fibrosis caused by exposure to aluminum metal was first seen in Germany before World War II and with increasing frequency during the war,18 but could not be demonstrated in either the United States or England until the reports of Mitchell et al in 1959 and 1961.174,175 Fibrosis in aluminum pot room workers was described by Shaver and Riddell,176,177 who reported disease in 10% of workers. Although sporadic reports of interstitial fibrosis with either metal or pot room exposure still appear,178–180 disease is rare, and certainly much less common than the figures produced by Shaver and Riddell. However, Jederlinic et al181 found a 1% incidence of interstitial fibrosis in a factory using alumina as an abrasive, and cases with at least radiographic fibrosis have been described by Akira.182 Other authors deny the existence of aluminum-induced fibrosis.183
Radiographically and pathologically, aluminum-induced interstitial fibrosis is predominantly upper and mid-zonal.174–184 Microscopic descriptions and illustrations are hard to interpret but show a pattern of diffuse interstitial fibrosis, and sometimes a more nodular process associated with aggregates of black dust.174,176,177,179 If dust is present in sufficient quantities, the lung may have a slate gray color on gross examination.177,179
The exact agent responsible for the fibrosing process is uncertain. Aluminum itself, either as particles or dissolved in lung fluid, stearin, and mineral oil used to coat the bare metal to prevent oxidation and aggregation of the particles, and gamma aluminum oxide and cristobalite evolved from the pots have all been proposed on the basis of human data or animal experiments174–187 (a detailed discussion is available elsewhere17). At this point the etiology of aluminum-induced fibrosis remains unresolved. Given the large population at risk, it is clearly not a common disease; however, the peculiar distribution of fibrosis, the association with specific types of exposure, and the animal data all suggest that either aluminum or some substance related to the processing of aluminum can produce fibrosis under certain unusual conditions.
A variety of other quite rare pathologic reactions have been seen in workers exposed to aluminum. In experimental animals large doses of finely divided aluminum powder have been shown to produce alveolar proteinosis,185,186,188 and a case of alveolar proteinosis in an aluminum rail grinder has been reported by Miller et al.189 The lung of this patient contained very large numbers of small (less than 1 μm) aluminum spheres. Herbert et al190 reported desquamative interstitial pneumonia in an aluminum arc welder; the lung tissue contained fine aluminum spheres similar to those found by Miller et al.189 Chen et al191 and DeVuyst et al192 described two cases of granulomatous lung disease in workers with exposure to aluminum welding and aluminum powder, and aluminum particles were demonstrated in the granulomas, but the disease may nonetheless have been sarcoidosis in both instances.
Airflow limitation has been reported in workers exposed to pot room fumes and aluminum salts,193–196 and it has been suggested that the incidence of lung cancer is also increased in pot room workers, although this has not been a universal finding.110 Given the large variety of irritating, sensitizing, and potentially carcinogenic compounds present in the pot room atmosphere, the role of aluminum itself remains uncertain.
Hard Metal Disease and Disease Caused by Cobalt
Hard metal (cemented tungsten carbide with cobalt) is a synthetic compound that approaches the hardness of diamond. It is used in tool bits, drills, and bearings that operate in conditions requiring strength and rigidity at high temperatures.197,198 Hard metal is prepared by heating finely divided tungsten and carbon to form tungsten carbide; then cobalt, and sometimes titanium, tantalum, or chromium carbide, are added, and the mixture fused at high temperature to form the final product. Fabrication generates a very fine dust with a mean particle size less than 2 μm.197,198 NIOSH estimated some 30,024 workers in the U.S. are exposed to hard metal.18 Most cases of hard metal disease have been described in production workers; however, disease is also found in those who file or grind hard metal tools for actual use, for example in some sawmill workers.199 The sensitizing agent, cobalt (see later) can be extracted from the metal by liquid lubricants used to cool the work piece, and aerosolized coolants are also a source of disease.200,201
Hard metal exposure can produce contact dermatitis, ARDS, occupational asthma, a syndrome resembling extrinsic allergic alveolitis (hypersensitivity pneumonia), and a distinctive form of interstitial fibrosis.201–204 The incidence of any of these conditions is unclear. When first reported in Germany, roentgenographic evidence of disease was seen in as many as 20% of factory workers.198 Current incidence rates appear to be much lower,197,198 although one report found a 31% incidence of abnormal chest films in a series of 41 hard metal workers.205 New cases continue to be reported182,204
Roentgenographically, interstitial fibrosis caused by hard metal is generally either lower or mid-zonal.197 Some reports depict ground-glass infiltrates on CT scan,182 whereas others describe CT scans with reticulation, traction bronchiectasis, and peripheral cystic spaces.206 The latter description does not fit with the pathologic picture seen in most cases of hard metal disease and is difficult to understand.
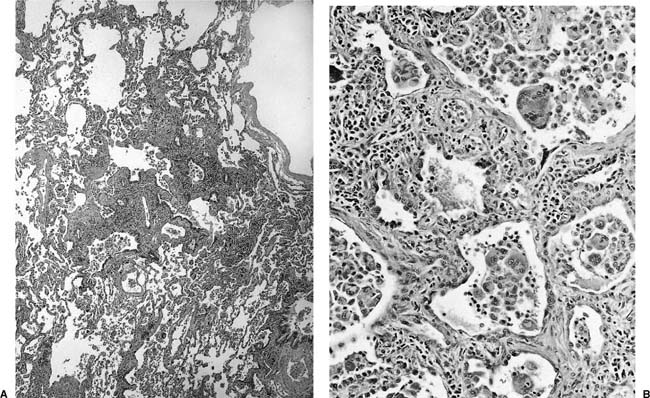
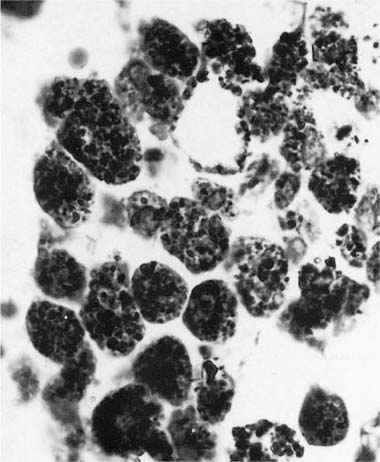
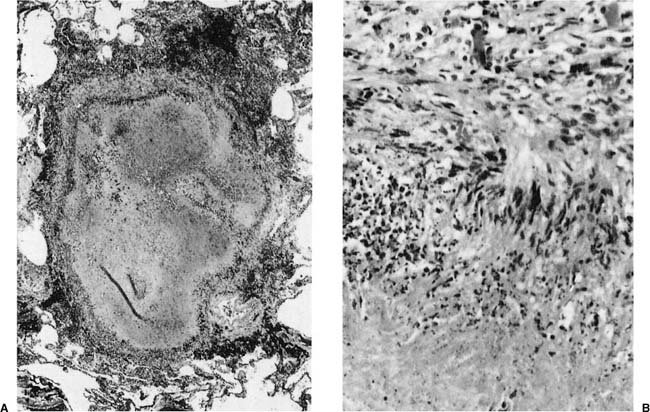
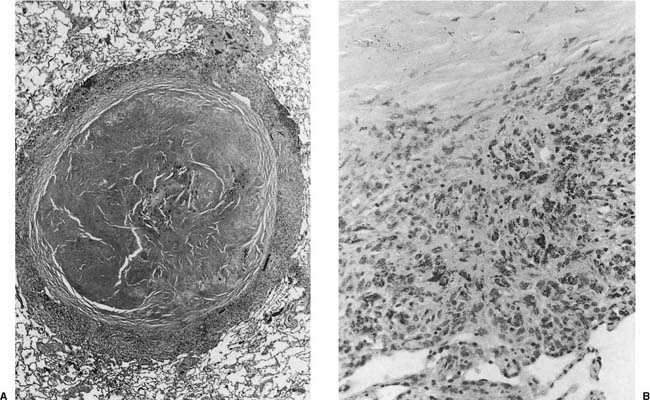
Histologically the typical picture is that of interstitial fibrosis and interstitial inflammation that is predominantly centrilobular in distribution, and an accompanying desquamative interstitial pneumonia (DIP) or a giant cell interstitial pneumonia (GIP)-like picture (Fig. 24–9), the latter characterized by a combination of ordinary alveolar macrophages and very large giant cells with relatively few and irregularly arranged nuclei in the airspaces.197–212 Although some believe that all cases of GIP actually represent hard metal disease,213 we have seen cases of GIP in which there was neither historical nor analytical evidence of hard metal exposure, and the reaction pattern should be regard as suggestive rather than specific. As well, some cases do not show a DIP- or GIP-type pattern.197–212,214–218 Honeycombing and diffuse severe fibrosis may be seen in very advanced cases.
Black particulate material can sometimes be found in macrophages and giant cells, but is never abundant, and in fact there is little on routine histologic examination to suggest dust-related disease. In cases where the history of exposure is uncertain, tungsten particles can usually be demonstrated by energy dispersive x-ray spectroscopy of histologic sections or dissolved tissue, and their presence confirms the diagnosis, because tungsten is never found as a background atmospheric contaminant in the general population. Particles of cobalt may also be found, but are less common because cobalt is soluble in tissue fluid.197,198,209 Exposure can also be confirmed by neutron activation analysis of pubic hair, nails, urine, or lavage fluid for tungsten, tantalum, or cobalt.210 Sensitization to cobalt can be demonstrated by lymphocyte proliferation tests, similar to those used to show beryllium sensitization.68
Sjogren et al200 reported three patients with interstitial infiltrates and decreases in airflow after exposure; in these patients signs and symptoms disappeared after removal from exposure, and reappeared, somewhat worse each time, on reexposure. The authors suggested that the process clinically resembled extrinsic allergic alveolitis. Hard metal exposure also causes occupational asthma, but this appears to occur in a subset of workers who do not develop interstitial lung disease.197,200–203,209,218
FIGURE 24–9 Low- (A) and high- (B) power views of a case of hard metal disease showing typical appearance of interstitial infiltrates, and filling of alveolar spaces by macrophages and giant cells.
Cobalt is probably the sensitizing agent for all of these reactions. Animal studies have shown that tungsten or tungsten carbide without cobalt produces relatively few and transient reactions, whereas cobalt, or tungsten carbide with cobalt, causes marked inflammatory responses, although none of these studies appears to reproduce the exact picture of hard metal disease in humans.198,219–222 Confirmatory evidence has been provided by a report of diamond polishers who use a polishing agent containing cobalt but not hard metal; these workers develop a syndrome that clinically appears similar to hard metal disease, and histologically shows a GIP-type reaction.223 As well, cobalt provokes asthmatic attacks in some patients with hard metal–induced occupational asthma.210 There appears to be a genetic susceptibility to hard metal disease: Potolicchio et al224 reported that disease is associated with residue glutamate-69 of the human leukocyte antigen (HLA)-DP chain. Lewis et al211 have shown that cobalt produces oxidant injury in experimental animal systems, and hydrogen peroxide–or hydroxyl radical–mediated lesions may well be important in the pathogenesis of hard metal disease.
Accurate diagnosis of hard metal disease is important because many (but not all) patients respond well to steroids and removal from exposure, particularly if exposure has been relatively short.68,197,209,200,223,225,226 Disease can progress after cessation of exposure.68
Cadmium
Although there is no doubt that acute high-level exposure to cadmium produces ARDS (and, potentially, renal impairment),17,23,26,28,31,227,228 claims that cadmium exposure produces chronic lung disease are controversial. Older studies reporting the development of emphysema in workers exposed to cadmium are flawed by a lack of information on smoking status, and the published descriptions and illustrations are those of ordinary smoke-induced centrilobular emphysema;229,230 however, some studies231 appear to show radiographic excesses of emphysema. Cross-sectional studies of cadmium workers have demonstrated only mild airflow obstruction, and that only in subjects with long-term high exposure.232 Snider et al233 claim to have produced emphysema in experimental animals using an aerosol of cadmium chloride, but other groups have been unable to reproduce this finding; indeed, some have reported interstitial fibrosis.234 Chawdhury and Louria235 found that cadmium reduced α1-antitrypsin levels in human plasma, but this observation has not been confirmed in studies of cadmium-exposed workers.236 At this point the evidence for cadmium-induced emphysema is inconclusive. It has also been claimed that cadmium-exposed workers have an increased mortality from lung carcinoma,112,113 an interesting observation, if true, because cigarette smoke is also a source of cadmium.237,238
Silicon Carbide
Silicon carbide pneumoconiosis is seen in those who manufacture silicon carbide (carborundum) abrasives.239,240 Pathologically the lesions are nonspecific and appear as both diffuse interstitial fibrosis and nodular fibrosis. Copious amounts of black dust may be present in the slides, as may ferruginous bodies with black fibrous cores (see discussion of ferruginous bodies in Asbestos-Related Disease, later). It has been suggested that silicon carbide manufacture is associated with an increased risk of lung cancer.119
Beryllium
Beryllium, a light metal with excellent thermal and electrical conductivity, is extracted from beryllium aluminum silicate (beryl ore); the empirical chemical formula is 3BeOAl2O36SiO2. It is mined in South America, Africa, Australia, and India. Beryllium oxide (BeO) is also obtained from bertrandite, a hydrated beryllium silicate mined in Utah.
Beryllium has many uses in advanced technology. Because of its lightness and strength, it is extensively used in the aerospace industry for structural components. Alloys of beryllium confer high resistance to fatigue. The high thermal conductivity of the oxide is employed in ceramics for the construction of heat shields for spacecraft and jet engine blades. Because it is nonmagnetic and transparent to x-rays, it is used in integrated circuits in the computer industry, in the manufacture of x-ray detection equipment, and in the atomic energy industry. A more comprehensive listing of sources of exposure is given in Table 24–16.
Beryllium disease was prevalent in industries using beryllium prior to 1950.241 After that time, recognition of the disease led to more stringent controls of exposure and the discontinuance of certain uses and practices, for example, manufacture of beryllium-containing fluorescent lamps, which carried a high risk of developing berylliosis.242 Nonetheless, between 1952 and 1978, 892 new cases of berylliosis were reported to the Beryllium Case Registry established at the Massachusetts General Hospital.241 Disease is still seen in 2 to 6% of exposed workers, and attack rates are higher in some specific occupations such as beryllium machining.68 It is also likely that some cases go unrecognized or are misdiagnosed as sarcoidosis.243 In 1986 NIOSH estimated that 807,024 people in the U.S. had potential beryllium exposure.1
Mining and extraction of beryllium |
Beryllium metallurgy |
Production and use of beryllium alloys |
Beryllium copper alloys: springs, electrical contacts, bearings, gears, precision castings, antispark tools, welding electrodes |
Beryllium nickel alloys: high-temperature springs, diamond drill bits, fuel pumps, dies for shaping |
Dental alloys: beryllium with nickel and chromium |
Other alloys: including aluminum, magnesium, and platinum |
Computers, integrated circuits, etc. |
Beryllium ceramics manufacturing |
X-ray tube window manufacturing |
Nuclear reactor manufacturing |
Guidance and navigation systems manufacturing (gyroscope housings) |
Aerospace manufacturing |
Gas mantle manufacturing |
Rocket fuel development research |
Salvage of fluorescent and neon lamps |
Foundry products and tool and die manufacturing |
Adapted from Sprince NL. Beryllium disease in occupational respiratory diseases. In: Merchant JA, ed. US Department of Health and Human Services (NIOSH), Publication No. 86–102. Washington, DC: NIOSH, 1986:385–400.
Clinical Features and Diagnostic Criteria of Berylliosis
Beryllium disease occurs in acute and chronic forms. The acute disease is a form of ARDS. The chronic disease is granulomatous and systemic, though the lung is the primary target organ. Beryllium exposure is also associated with an acute dermatitis, acute conjunctivitis, and acute nasopharyngitis.242
Patients with the acute disease present with rapid onset of dyspnea, cough, and substernal pain, with diffuse or localized infiltrates on the chest radiograph. The illness follows shortly after exposure, and its severity is proportional to the exposure concentration. There is a mortality of approximately 10%, and a further 10% of cases may progress to chronic berylliosis.242
Chronic beryllium disease, by contrast, may present years or months after cessation of exposure to beryllium. There is a general dose-response relationship, but disease is also seen in individuals with very low exposure who are not directly employed in handling beryllium products, for example secretaries and other front-office workers in beryllium processing facilities.68 Chronic beryllium disease may be asymptomatic and be found on a routine chest radiograph, or the patient may present with exertional dyspnea and signs of chronic restrictive or obstructive lung disease. The disease follows a variable clinical course; for some patients, the disease may spontaneously regress, others respond to corticosteroid therapy, and for the minority the disease may progress to respiratory failure.
Because exposure to beryllium may not always be obvious, and because the disease resembles sarcoidosis in many respects, detailed clinical/pathologic criteria for establishing the diagnosis of chronic beryllium disease have been developed by the Beryllium Case Registry.244 They are listed in Table 24–17.
1. Establishment of beryllium exposure: a. Based on occupational history b. Based on analysis of tissues or urine for beryllium 2. Objective evidence of lower respiratory tract disease and a clinical course consistent with beryllium disease 3. Chest x-ray films with radiologic evidence of interstitial fibronodular disease 4. Evidence of restrictive or obstructive defect or diminished carbon monoxide diffusing capacity 5. Pathologic changes consistent with beryllium disease on examination of lung tissue and/or thoracic lymph nodes 6. Evidence of hypersensitivity to beryllium (usually measured as beryllium-induced lymphocyte proliferation) |
Adapted from Kelleher P, Pacheco K, Newman LS. Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect 2024;108(suppl 4):685–696, Churg A, Colby TV. Diseases caused by metals and related compounds. In: Churg A, Green FHY, eds. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998:77–128; and Kazemi H. Beryllium disease. In: Last JM, ed. Preventive Medicine and Public Health, 11th ed. New York, Appleton Century Croft, 1980.
Pathology of Acute Beryllium Pneumonitis
This is now a rare condition. It presents as ARDS and shows the histologic features of diffuse alveolar damage. Acute bronchitis and bronchiolitis are also present. Microscopic examination shows formation of hyaline membranes with alveolar edema and hemorrhage. Granulomas do not develop in the acute form of the disease.
Pathology of Chronic Beryllium Pneumonitis
Chronic beryllium disease is characterized by the formation of noncaseating granulomas, which in appearance and distribution are identical to those seen in sarcoidosis (Fig. 24–10). Thus granulomas may follow lymphatic routes along the airway, may be found in the alveolar interstitium, and may coalesce to produce nodular lesions. The granulomas may disappear with time, leaving nonspecific honeycomb fibrosis or nodular scars; the latter are found in 40% of cases and often show central calcification or necrosis.245 The presence of granulomas with slight or absent interstitial cellular infiltration and fibrosis is apparently associated with a better prognosis and greater response to steroid treatment. Granulomas may also be found in hilar lymph nodes and in extrapulmonary sites including the skin, liver, spleen, pancreas, kidney, adrenals, bone marrow, muscle, and central nervous system, but apparently not in the myocardium.
The histologic features of chronic beryllium disease are nonspecific. Although sarcoidosis is the major clinical and histologic confounder,243 extrinsic allergic alveolitis, lymphocytic interstitial pneumonia (LIP), silicosis, and granulomatous infections should be considered in the differential diagnosis.242 In the final analysis, a definitive diagnosis can only be made in the light of both clinical and laboratory based information (Table 24–17).
Beryllium Exposure and Lung Cancer
Beryllium has been established as a powerful pulmonary carcinogen in experimental animals. However, the evidence for beryllium as a human pulmonary carcinogen is contradictory, and is confounded by the question of whether an increased risk for lung cancer (assuming it exists) reflects beryllium itself or beryllium-induced lung fibrosis.80,83,87,111,246
Pathogenesis and Tissue Analysis for Beryllium
The pathogenesis of the acute disease appears to result from a direct toxic effect of the beryllium fumes on the airway mucosa and alveolar-capillary membrane. The chronic disease is an immunologic disorder characterized by a type IV hypersensitivity response to beryllium, which appears to act as a hapten.68 This view is supported by animal experimental studies and the presence in the blood from patients with berylliosis of lymphocytes sensitized to beryllium.247–249 It has been reported that the presence of HLA-DP 1 glutamate-69 is a susceptibility marker for chronic beryllium disease,250 as it is of hard metal susceptibility.
FIGURE 24–10 Granulomas, some containing Schaumann’s bodies, in a case of chronic berylliosis.
In vitro beryllium-induced lymphocyte transformation (beryllium lymphocyte proliferation testing) is a valuable adjunct to diagnosis and for population screening;251 however, although these tests show beryllium sensitization, it is as yet unclear whether they necessarily indicate the presence of disease.248 A variety of chemical techniques are available for documenting the amount of beryllium in lung tissue.242 For traditional chemical (atomic absorption spectroscopy and related techniques), related large amounts of tissue, typically upward of 1 g, are required. The amount of beryllium in the lung varies widely, ranging from 0.004 to 45.7 g/g in the lung of patients with chronic beryllium pneumonitis.243 Patients with acute berylliosis usually have higher levels, whereas nonexposed individuals and patients with sarcoidosis usually have less. Tissues other than lung may be analyzed for their beryllium content and the presence of beryllium in urine can also be used as an indication of exposure.252 Butnor et al253 have recently reported the use of atmospheric thin window energy dispersive x-ray spectroscopy to detect beryllium particles in the granulomas in tissue sections. This approach is potentially very useful because it can be applied to paraffin-embedded material.
Coal Workers’ Pneumoconiosis and Related Diseases
Historical Considerations
The first morphologic description of coal workers’ pneumoconiosis (CWP) was of black fibrotic lesions with cavitation in the lungs of British Coal Miners in 1831.254 For almost a century thereafter, the disease was considered to be due to silica exposure in the coal mines. This view was challenged by Collis and Gilchrist255 when they reported pneumoconiotic changes in the lungs of coal trimmers who shoveled coal that had been washed relatively free of silica. It was reinforced by other similar studies256 and by the demonstration of a pneumoconiosis similar to that seen in coal workers in the lungs of workers exposed to artificial graphite, carbon black, and lamp black, which are virtually free of silica.257 The lesions in the lungs of coal miners, however, may be considerably modified by the presence of high concentrations of silica and other minerals, and approximately 10% of U.S. coal miners have evidence of silicosis at death.258
The diseases associated with coal mining are listed in Table 24–18. CWP, which encompasses simple and complicated forms, is the most commonly diagnosed condition. It is important to recognize that CWP is not synonymous with the term black lung. The latter is a legal definition that includes, in addition to CWP, other chronic lung conditions related to employment in the coal mines.259 Therefore, black lung would encompass chronic bronchitis, emphysema, and silicosis when these can be causally linked to employment in coal mines.
Coal workers’ pneumoconiosis: |
Simple: macules, nodules |
Complicated: progressive massive fibrosis |
Rheumatoid pneumoconiosis |
Silicosis |
Interstitial fibrosis |
Chronic bronchitis (industrial bronchitis) |
Emphysema |
Coal and Coal Mining
Coal is the world’s most abundant fossil fuel resource. Coal deposits underlie approximately 14% of the contiguous land area of the United States with approximately 396 billion metric tons of reserves that can be mined economically for several centuries at current rates of production. The number of miners employed in the U.S. has declined in recent decades, and currently there are approximately 150,024 active coal miners in the U.S.1
Underground coal is extracted by two methods: board and pillar mining, and long-wall mining.260 When the coal is close to the surface, strip or open cast mining is the most efficient method, and approximately half of U.S. coal production is from the latter source. Dust exposure is generally low in surface mines, and this is reflected by a low prevalence of pneumoconiosis.261 Surface drillers, however, may be exposed to high concentrations of silica and develop silicosis.262,263 In underground mining the greatest concentrations of respirable dust are found at the coal face. Motormen and roof bolters are exposed to higher concentrations of silica than face workers and are more likely to develop classical silicosis.258,264,265
Coal is derived from compressed organic material, including wood, bark, roots, leaves, spores, and seeds in sedimentary strata. Coals are ranked according to their geologic age into young coals, such as lignite, which are relatively soft and have a high moisture content, and older coals, such as anthracite, which are black, hard, and glassy with low moisture content and high fixed carbon.266 Rank has been shown to be a factor in the toxicity of coal. In general, the higher ranked coals are associated with a greater prevalence and severity of pneumoconiosis.264,265,267
Coal is a mineralogically complex material. In addition to its carbonaceous content it contains variable amounts of minerals and trace elements. The major mineral inclusions in coal are alumino-silicates (clays), sulfides, carbonates, silica, and feldspars.266 In addition, trace amounts of organic compounds such as benzene, cyclohexane, cyclohexanone, methylene, naphthalene, phenol, phenyl ether, polymethylene, phenanthrene, and dihydroaromatics are all present in varying amounts.268
Coal mine dust consists of a mixture of suspended particulates derived from the coal seam, adjacent rock strata, rock dust used to dampen explosions, and equipment (for example, diesel engines and welding) used in the mines. Coal mine dust usually contains a greater mineral fraction than the coal seam, and the silica fraction is relatively enriched.269 Other potential respiratory hazards in underground coal mining include oxides of nitrogen produced during shot firing, and fumes released from electrical fires, welding, and brazing operations. Radon daughters are released from the rock strata during the mining of coal, generally in low concentration.270 Some coal deposits in Missouri, southern Iowa, and the lignite deposits in South Dakota may contain the radioactive nuclides 238U, 235U, and 232Th.
In the United States the permissible levels of respirable dust in underground mines were established in the Federal Coal Mine Health and Safety Act of 1969 and its subsequent amendments.271,272 Since 1972, this limit has been set at 2.0 mg/m3, with a quartz content in the dust not to exceed 5%.
Clinical and Radiographic Features of Coal Workers’ Pneumoconiosis
Simple CWP is classified according to the number, size, and shape of small opacities on the chest radiograph using a system of standard reference films developed by the ILO.273 The most common radiologic abnormality is multiple, small, rounded opacities in the upper zones of both lungs. The category of pneumoconiosis has been shown in several large epidemiologic studies to be related to the intensity and duration of exposure to coal mine dust.274,275 In general, there is a good correlation between pathologic grade of pneumoconiosis and radiographic findings.276,277 This correlation is increased in studies using high-resolution CT.278
Miners with the milder forms of simple CWP usually have no symptoms, although longitudinal studies of pulmonary function suggest that some proportion of such individuals will have abnormal pulmonary function tests and airflow obstruction;126–129 furthermore, more sensitive tests of small airway function8 and the ratio of ventilation to perfusion may reveal abnormalities in coal miners with dyspnea.9 As well, some miners develop chronic cough and expectoration of black-tinged sputum. This condition, known as industrial bronchitis, is thought to be a reaction of the airways to the larger dust particles and is seen in miners with and without radiographic evidence of CWP. More information on the clinical and epidemiologic features of CWP is available elsewhere.264,267,274,279–283
By contrast, miners with complicated CWP (progressive massive fibrosis, PMF) often have pulmonary impairment, and PMF may be associated with premature death.284,285 PMF usually develops from a background of severe simple CWP and is associated with chronic airflow obstruction, restrictive defects, uneven ventilation/perfusion, reduced diffusing capacity, and low PaO2, leading often to pulmonary hypertension and cor pulmonale. The prevalence of CWP has declined in the U.S., Europe, and Australia in recent decades.260,282,283 However, mortality associated with CWP increased in younger miners (15 to 44 years of age) in the period 1985 to 1996,281 and new cases of pneumoconiosis are occurring in miners who have worked exclusively under the current dust exposure limits.282
PMF may be confused with carcinoma, tuberculosis, and mycotic infections because these conditions also produce large radiographic opacities with cavitation. Tuberculosis, other mycobacterial infections, and aspergillosis may be found in association with PMF.286 The bilaterality of PMF with a background of nodular opacities are useful features in distinguishing PMF from carcinoma. Open lung biopsy is rarely required to establish the diagnosis of PMF, and is almost never indicated for the diagnosis of simple CWP.
Pathogenesis
The primary risk for CWP is related to the cumulative exposure to dust (years of exposure × exposure concentration). Four basic mechanisms are thought to underlie the pathogenesis of CWP: (1) direct cytotoxicity, (2) activation of oxidant production by lung phagocytic cells, (3) release of proinflammatory cytokines from lung cells, and (4) secretion of growth factors.287–289 Regional factors and the composition of the dust have been shown to be of importance. Dusts with higher silica content are associated with greater risk for CWP,258 as are dusts with greater amounts of bioavailable iron.290,291 These dusts are able to drive the Fenton reaction resulting in the enhanced generation of reactive oxygen species (ROS). Coal and silica dust also have intrinsic surface free radicals, and these too may be important in the pathogenesis of diseases caused by these dusts.292,293 Recently, attention has focused on genetic factors for increased susceptibility to develop pneumoconiosis. Several promising candidate genes have been identified.294,295
Pathologic Features of Coal Workers’ Pneumoconiosis
Disease Prevalence
Despite the heterogeneity in composition of coal mine dust, it is now generally accepted that the lesions of CWP are specific in nature and occur in coal workers exposed to coal mine dust irrespective of mining region or country of origin. The prevalence of CWP at death has been determined in a large population of autopsied miners submitted to the U.S. National Coal Workers Autopsy Study between 1971 and 2024.276 The program is voluntary and is done at the request of next of kin in order to facilitate black lung entitlements. Pathologists are paid a fee for conducting the autopsy and for submitting pathologic and demographic details to NIOSH.296 Although the payment of fees and the seeking of compensation may produce a selection bias, data from this group of workers constitute the largest single source of pathologic information on CWP in North America.
Number | 3365 |
Mean age (years) at death | 62 ± 12 (SD) |
Average years worked in underground mines | 26 ± 14 (SD) |
Race/sex | 94% white males |
Cigarette smokers (ever) | 74% |
Average pack year consumption for smokers 24 ± 20 (SD) |
|
The demographic features of this population of miners are shown in Table 24–19, and the prevalence of pneumoconiotic lesions by type in Table 24–20. The high prevalence of simple CWP in these workers reflects the fact that this is an older population with long job tenures, many of whom received their exposures during periods when dust levels were high. By contrast the prevalence of CWP in coal miners who received their coal mine dust exposure under the current limits is lower.282
Pleural and Lymphatic Changes
Coal mine dust accumulates in the subpleural connective tissues, interlobular septa, and pleural lymphatics in coal miners. The pleural surfaces are often deeply black pigmented, with accentuation of the pigmentation corresponding to the junctions of the interlobular septa and the pleura. Pigmentation is usually greatest in the upper zones of the lung.
The peribronchial, hilar, and associated lymph nodes are usually enlarged and densely black on cut surface. Microscopic examination shows large numbers of pigmented histiocytes in the sinusoids and perifollicular areas. Silicotic nodules are also frequently observed in these lymph nodes (Fig. 24–11) and usually occur in the absence of silicotic nodules in the parenchyma258 (Table 24–20). There is evidence for selective enrichment of quartz in the lymph nodes.297,298 Black pigment and silicotic nodules may also be observed in extrathoracic sites, particularly the abdominal lymph nodes, liver, and spleen.299
Type of Pneumoconiosis | Percent |
|---|---|
Macules | 45.6 |
Macules and focal emphysema | 35.8 |
Nodules | 18.9 |
Progressive massive fibrosis | 5.5 |
Silicosis | 12.6 |
Silicotic nodules in lymph nodes | 52.9 |
Coal Dust Macules
Coal dust macules are soft lesions ranging in size from 0.5 to 6 mm in diameter centered on respiratory bronchioles. They are often associated with a variable degree of airspace enlargement, termed “focal emphysema” around the macule. The density of the macules is greatest in the upper zones of the lung but may involve all regions (Fig. 24–12). Similar lesions may be observed in association with exposure to lignite and other carbonaceous dusts, in heavy cigarette smokers, and in the lungs of residents of heavily polluted urban centers. However, the coal dust macule can be distinguished from these other types of macule by the distinctive features of the dust. Bituminous coal, when viewed under intense illumination at high magnification, appears as translucent golden brown particles with angular borders.300 Combustion products of cigarettes, fossil fuels, or biomass are opaque, black, smaller, and have rounded borders.
FIGURE 24–11 Silicotic nodule in tracheobronchial lymph node of coal miner. The nodules are histologically identical to those seen in the parenchyma. A diagnosis of silicosis cannot be made on lymph node histopathology alone (see text).
FIGURE 24–12 (A) Whole lung section from coal miner from West Virginia showing coal dust macules with mild focal emphysema. The greatest density of macules is seen in the upper zones. (B) Close-up view of upper lobe showing relationship of macules to focal emphysema. (C) The chest roentgenograph shows no evidence of pneumoconiosis. Note: Mild forms of coal workers’ pneumoconiosis may not be seen on the chest film due to the relative insensitivity of the radiograph compared to the pathologic specimen.
FIGURE 24–13 (A) Low-power view of coal miners’ lung showing macules (thin arrows) and micronodules (thick arrows). The lesions are seen in the walls of respiratory bronchioles. (B) Higher power view of macule shown in inset.The lesion consists of pigmented macrophages in a reticulin stroma within the walls of second- and third-order respiratory bronchioles.
Microscopically, the coal dust macule is composed of coal dust–containing macrophages and histiocytes in a reticulin stroma in the walls of respiratory bronchioles (Figs. 24–13 and 24–14). All three orders of respiratory bronchioles are affected, and the lesion may extend into the adjacent alveolar septa. Active miners also show accumulation of black pigmented macrophages within alveoli adjacent to respiratory bronchioles (Figs. 24–15 and 24–16). Collapse of the alveolar walls around the macrophages and their incorporation into the interstitium may be important factors in the formation of the coal dust macule. Over time the macules become more collagenized (fibrotic) and come to resemble the micronodules described below.
The zone of enlarged airspaces around the macule (i.e., focal emphysema) is variable in extent (Figs. 24–13 and 24–14). Focal emphysema is a form of centriacinar emphysema. It appears to develop in parallel with the macule, and its presence is considered necessary for the pathologic diagnosis of simple CWP.301 It is seen in both smoking miners and in miners who have never smoked. Microscopically the initial stages are characterized by dilatation of respiratory bronchioles with atrophy of bronchiolar smooth muscle.302 Later destructive changes common to all forms of emphysema occur with fragmentation of elastic fibers, loss of septal capillaries, and the development of abnormal fenestrations.
Coal Dust Nodules
Coal dust nodules are usually seen against a background of the more numerous macules. Like macules, they tend to be more common in the upper zones and may be centered on respiratory bronchioles; however, they are also frequently seen in the interlobular septa and in the subpleural and peribronchial connective tissues (Fig. 24–17). Unlike macules, nodules are firm to palpation. Their borders are usually rounder than those of macules but they also may show serpiginous borders and be surrounded by enlarged airspaces. The latter is termed irregular or scar emphysema. Their size is variable, but they are usually larger than the macules. When they are greater than 1 cm in diameter, they should be classified as PMF. This ensures uniformity of classification with the ILO radiographic classification system273 and with black lung legislation.276
Microscopically, coal dust nodules consist of dust-laden macrophages in a fibrotic stroma. The latter is composed of collagen and reticulin arranged in interlacing bundles (Figs. 24–18 and 24–19). Nodules with concentrically arranged collagen are indicative of high quartz exposure (Figs. 24–19 and 24–20). Degenerative changes are common in nodules; these include cholesterol clefts, cavitation, calcification, and vascular obliteration.
FIGURE 24–14 Characteristic coal dust macules in walls of respiratory bronchioles. Note the marked focal emphysema surrounding the macules.
FIGURE 24–15 Section of lung from miner killed in a mining accident. Note large numbers of pigmented macrophages in the alveoli. Collapse of the alveolar walls around the macrophages with incorporation of the dust into the interstitium is one possible mechanism for the formation of the macule. (From Churg A, Green FHY. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998, with permission.)
The differential diagnosis of coal dust nodules includes silicotic nodules, rheumatoid nodules, and infectious granulomata. Silicotic nodules and rheumatoid nodules in coal miners are described below. Healed and pigmented infectious granulomata may pose a difficult diagnostic problem. Special stains are unlikely to reveal organisms. Granulomata usually contain less pigment than coal dust nodules and are more likely to be calcified. They also tend to have a random and sparse distribution in the lung and may not be associated with other lesions (for example, macules). A single pigmented fibrotic lesion in a coal miner is unlikely to be CWP.
Progressive Massive Fibrosis
Progressive massive fibrosis (PMF, also referred to as complicated CWP), usually but not always appears on a background of severe simple CWP. Because PMF is associated with disability and premature death, major efforts have been made to prevent the development of PMF in coal mining populations. Unfortunately, the factors that determine the progression of simple CWP to the complicated form are not fully understood. Several factors have been shown to be associated with an increased risk of developing PMF: cumulative dust exposure, the proportion of quartz in the coal mine dust, immunologic abnormalities, and infectious agents.286,300,303–306 Many cases, however, develop in the absence of these factors.232
PMF is a disease that can progress in the absence of further exposure. Moreover, the younger the age of onset, the more rapid the progression.284 Because PMF is usually a compensable condition, it is important to be aware of the legal definition of this condition. Black lung legislation in the United States defines PMF as an opacity or fibrotic pneumoconiotic lesion 1 cm in diameter or greater. It is important, therefore, to accurately measure the size of lesions that may conform to this definition.
PMF lesions are most commonly observed in the upper and posterior portions of the lung. They are usually bilateral, which aids in their distinction from lung tumors on the chest radiograph. Their shape varies; they may be round, oval, or irregular, but are usually well demarcated from the surrounding lung (Fig. 24–21). They tend to transgress anatomic boundaries, leading to destruction of the bronchovascular structures and obliteration of interlobular fissures. With time, lesions of massive fibrosis tend to contract toward the hilum, causing distortion of the bronchovascular rays on the chest radiograph (Fig. 24–22). The cut surface of the typical lesion is rubbery in texture and may show cavities of varying sizes containing semifluid black contents that can scintillate due to the presence of cholesterol crystals. The lesions should be cultured for mycobacterial and fungal organisms. Calcification and silicotic nodules may also be observed within the massive lesions.
FIGURE 24–16 High-power view of coal dust particles within alveolar macrophages. The angular shape of the particles, their translucency, and their golden to dark brown color are characteristic of bituminous coal mine dust. These features distinguish the particles from carbonaceous combustion products. (From Churg A, Green FHY. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998, with permission.)
FIGURE 24–17 Whole lung section showing micro- and macronodules. The nodules predominate in the upper lobes and posteriorly. The nodules with pale centers are more likely to have a silicotic appearance on microscopy.
Microscopically, PMF lesions are composed of loose dust, collagen, and macrophages. Extensive areas of necrosis and cavitation are seen, and cholesterol crystals are frequently observed in the clefts and cavities within the lesion. Outlines of coal dust or silicotic nodules are seen in many of the lesions of PMF (Fig. 24–23). In addition, fragments of cartilage and elastic fibers indicative of residual and destroyed bronchovascular structures are present. Airways adjacent to the lesion are usually markedly distorted. Emphysema appears to be more severe in miners with PMF and may contribute to the pulmonary impairment.307 Vascular changes are seen in almost all PMF lesions and involve arteries, veins, and lymphatics. The lesions tend to be destructive with infiltration of the walls of the vessels by pigmented macrophages, leading to fibrosis and eventual obliteration (Figs. 24–23 and 24–24). A similar histologic pattern may be seen in peribronchial lymph nodes, but these lesions can be distinguished from true PMF by the presence of lymphoid tissues at their periphery.
Rheumatoid Pneumoconiosis (Caplan’s Syndrome)
This severe and rapidly progressive form of complicated pneumoconiosis was originally described by Caplan in Welsh coal miners with rheumatoid arthritis.308 Subsequently, it was found to occur in miners with circulating rheumatoid factor but without arthritis and in individuals exposed to silica.309 The condition appears to be rare in North America.310
Characteristic radiographic features are the presence of rapidly enlarging, circumscribed nodules ranging in size from 0.3 to 5 cm in diameter. Unlike PMF, the condition usually arises against a background of mild simple CWP. The nodules are paler and more yellow than PMF lesions and show concentrically arranged dark and light pigmented bands. They may also show central cavitation and calcification. Caplan’s nodules typically are larger and softer than silicotic nodules.
FIGURE 24–18 Micronodules. The three nodules show varying degrees of collagenization. The circular orientation of the hyalinized collagen in the nodule at top is indicative of a high silica content. Random orientation of the collagen fibers as seen in the micronodule at bottom right is more characteristic of the micronodule of coal workers. (From Churg A, Green FHY. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998, with permission.)
FIGURE 24–19 Micronodule. This lesion is centered on a respiratory bronchiole, indicating that it may have originated within a macule. It is distinguished from a macule by its greater collagen content, which would make it palpable on gross examination. Note the collections of dust within macrophages at the periphery of the lesion, indicating that the miner was actively employed at the time of the death.
Microscopically, the lesions are identical to rheumatoid nodules occurring in the lungs of nonoccupationally exposed individuals with the exception of rings of coal dust at the periphery (Fig. 24–25A). There is a central zone of eosinophilic necrotic tissue surrounded by a layer or layers of palisaded fibroblasts and macrophages (Fig. 24–25B). Giant cells may be seen but are less common than in an infective granulomata. The periphery of the lesion is surrounded by collagen fibers with variable numbers of lymphocytes, plasma cells, and macrophages. Immunoglobulins, including rheumatoid factor, have been demonstrated immunohistochemically in the lesions of rheumatoid pneumoconiosis.305
The differential diagnosis includes tuberculosis and other infective granulomata such as atypical mycobacterial infections and histoplasmosis. Differentiation can usually be made on the basis of special stains and culture of the fresh lesion, as well as by the results of laboratory tests for rheumatoid factor.
Silicosis in Coal Workers
Silica exposure has long been recognized as a respiratory hazard in coal mining, and workers in certain job categories within the mines such as roof bolters, motormen, and tunnel drillers are at high risk for silicosis.258,311 Brief exposure to high concentrations of silica is also a risk factor for silicosis.312 It is now recognized that the pathologic features of silicosis are distinct from those of CWP, although the radiographic appearances of the two diseases may be identical. Silicotic lesions are seen in the lungs of approximately 13% of U.S. underground coal miners (Table 24–20), and usually occur against a background of simple nodular and macular CWP.258 Experimental studies and analysis of lung dust in coal miners at autopsy indicate that the proportion of quartz in the inhaled dust or dust retained in the lung needs to exceed a certain threshold (~18%) before the characteristic nodules of silicosis develop.313,314
FIGURE 24–20 Section of lung from coal miner showing confluent nodules. Several of the nodules have concentrically arranged collagen indicative of high silica content. Histologically, this lesion is identical to progressive massive fibrosis (PMF), and should be classified as such if it measures greater than 1 cm in greatest dimension.
FIGURE 24–21 Progressive massive fibrosis. Whole lung section showing large black cavitating lesions in the upper and lower lobes of the lung. The lung elsewhere shows nodules and macules of simple coal workers’ pneumoconiosis (CWP).
Macroscopically silicotic nodules may be indistinguishable from the nodules of CWP but may have paler centers. Silicotic nodules may coalesce to form lesions of conglomerate silicosis (PMF), and the distinct outlines of silicotic nodules can often be seen within the large areas of fibrosis.
The diagnosis of silicosis in coal workers is made by microscopic examination. The mature silicotic nodule has a similar appearance to that observed in non–coal workers (see Silicosis and Other Diseases Caused by Silica, later) and is composed of concentrically arranged laminated bundles of mature collagen surrounding a hyalinized or partially necrotic and calcified center (Figs. 24–26 and 24–27). The periphery is usually more cellular with macrophages containing coal mine dust in a loose reticulin stroma. Polarized light microscopy can be of diagnostic value. Particles of crystalline silica are cuboidal in shape and show weak birefringence. Silicate minerals, which might also be found in coal dust, are brightly birefringent and have a needle or plate-like morphology.
Silicotic nodules are also frequently observed in the tracheobronchial lymph nodes (Table 24–20) and may occur in the absence of parenchymal silicosis. However, the diagnosis of silicosis should be based entirely on the presence of parenchymal disease. The acute and accelerated forms of silicosis are rarely seen in coal workers.
Diffuse Interstitial Fibrosis in Coal Miners
Diffuse pulmonary fibrosis has been reported in up to 18% of coal miners at autopsy in both the United States and Great Britain.315 It is associated with irregular opacities on the chest radiograph and a relatively benign clinical course.316 Some cases develop a more severe form of interstitial fibrosis comparable to idiopathic pulmonary fibrosis (IPF).317,318 Severe cases show macroscopic honeycombing. Histologically, the inflammatory infiltrate is highly variable as is the amount of dust within the fibrotic interstitium. It is important to be aware of this entity, as many cases are inadvertently diagnosed as IPF. Immunologic factors may be involved, as this entity occurs more commonly in coal miners who have rheumatoid pneumoconiosis.267 The role of silica and silicates in coal mine dust in the pathogenesis of these lesions has not been established.
FIGURE 24–22 Progressive massive fibrosis. (A) Whole lung section showing multiple opacities in both upper and lower lobes, that measure greater than 1 cm across. The lung also shows severe panacinar emphysema. (B) Chest roentgenograph from the same case showing large opacities in the right upper lobe with distortion of the bronchovascular markings on that side. The widened intercostal spaces, flattened diaphragm, and hypolucency of the lower lung fields are indicative of emphysema. (From Churg A, Green FHY. Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998, with permission.)
Emphysema and Chronic Bronchitis in Coal Miners
Clinical and epidemiologic studies have consistently demonstrated that exposure to coal mine dust causes cough and sputum production, and abnormalities of ventilatory capacity in both smoking and nonsmoking miners.126–129,279,280,319–322 The relative importance of cigarette smoking and coal mine dust in reducing ventilatory capacity has also been addressed in several of these studies. The contributions of smoking and coal mine dust to loss of ventilatory capacity are comparable in amount and additive in effect. A longitudinal epidemiologic study of a large cohort of British coal miners showed that, in addition to dose-dependent effects of coal mine dose on lung function, there may be subgroups of miners who are unusually susceptible to the effects of the dust.322 This has been confirmed in U.S. coal miners.323
Although there is general agreement that exposure to coal mine dust produces limitations in airflow,126–129,320 there is less agreement concerning the relative contribution of small airway disease (i.e., the coal macule77) and emphysema to the obstruction77 (see discussion of this topic in Disease Caused by Inhaled Gases and Fumes, earlier in chapter). Autopsy studies of coal miners have shown that coal mining carries a risk for emphysema that is additional to the effects of age and cigarette smoking. Specifically, these studies have shown significant differences in emphysema scores between coal miners and age- and sex-matched nonminer controls,323–327 and positive correlations between the emphysema score and years of underground mining,328 x-ray category of pneumoconiosis,328 pathologic grade of pneumoconiosis,324,329,330 exposure to respirable dust,330 and lung dust content.331,332 A study from NIOSH involving a cohort of 266 coal miners from southern West Virginia333 showed a significant positive relationship between years of underground mining and emphysema index for both smoking and nonsmoking coal miners (Table 24–21). The effect of average cigarette smoking on the emphysema index was approximately equal to the effect of average coal mine dust exposure.
FIGURE 24–23 Progressive massive fibrosis. Section shows pigmentation, fibrosis, cavitation, and obliteration and distortion of blood vessels (thin arrows) and a large cartilaginous airway (thick arrow).
FIGURE 24–24 Progressive massive fibrosis. Section shows muscular pulmonary artery within the PMF lesion. There is fibrosis of the intima, and the wall is infiltrated by pigmented macrophages.
Autopsy studies also indicate that centriacinar emphysema (including focal) is the most common type of emphysema in coal workers, although panacinar, irregular, and paraseptal emphysema are also seen.276,333 The pathogenesis of emphysema in coal miners appears to proceed by a mechanism similar to that shown for cigarette smoking and involves excessive release of proteases and ROS and inhibition of defense mechanisms.289,334–337
Very few morphologic studies of chronic bronchitis have been conducted on coal miners. Two of these studies gave ambiguous results;328,338 however, one study of 94 autopsied U.K miners did show the expected correlation between the Reid index and both smoking and cumulative exposure to coal mine dust.339 It may be that morphologic indices of chronic bronchitis (bronchial mucous gland enlargement and mucous cell metaplasia) are relatively insensitive indicators of chronic bronchitis, as almost all clinical and epidemiologic studies have shown chronic mucous hypersecretion and cough associated with exposure to coal mine dust.
Although the published data in this area are consistent, it is important to recognize the limitations of postmortem retrospective studies. These include sample selection biases, inadequate information on smoking and exposure, and the use of less than optimal lung samples (e.g., uninflated lungs).
Cancer in Coal Workers
The majority of coal miner mortality studies have shown that lung cancer is slightly less common in coal miners than in comparable populations.285,340 Most lung cancers in coal miners can be accounted for by the effects of cigarette smoking. An autopsy study of 171 cases of lung cancer in U.S. coal miners showed that the majority of tumors were centered on large airways and were more commonly observed in the upper lobes.341 The distribution by cell type was squamous cell 30%, adenocarcinoma 27%, small cell undifferentiated 26%, large cell undifferentiated 9%, and others 8%. Of the miners with lung cancer, 95% were current or ex-cigarette smokers. No effect of years of mining was observed on cell type distribution. It was concluded from this study that the cell type and regional distribution of the tumors were characteristic of those reported in male cigarette smokers in the general population.
By contrast, coal miner mortality studies have consistently shown an excess of deaths due to stomach cancer (reviewed elsewhere275). The causes for this are unknown, though it should be noted that miners often chew tobacco underground due to the ban on underground smoking.
FIGURE 24–25 Rheumatoid pneumoconiosis. (A) The large nodule shows central amorphous necrosis with bands of pigmented histiocytes at the periphery. (B) The edge of the same lesion showing palisaded histiocytes sandwiched between the amorphous necrosis and the fibroblastic and histiocytic cellular reaction at the periphery. (From Churg A, Green FHY: Pathology of Occupational Lung Disease, 2nd ed. Baltimore: Williams & Wilkins, 1998, with permission.)
FIGURE 24–26 Silicotic nodule in coal workers’ lung. The center of the nodule is composed of hyalinized, concentrically arranged collagen fibers surrounded by a serpiginous mantle of coal dust containing macrophages in a reticulin matrix. Note the peripheral (scar) emphysema associated with the lesion.
FIGURE 24–27 Silicotic nodule in coal miners’ lung. Higher power view of typical silicotic nodule showing central necrosis and pigmentation surrounded by concentrically arranged hyalinized collagen fibers.
Sandblasting, grinding and use of abrasives |
Mining, quarrying, and tunneling |
Foundry work and boiler scaling |
Manufacture of ceramics, and enameling |
Manufacture of silica containing fillers |
Farming |
Silicosis and Other Diseases Caused by Silica
Historical Considerations
Silicosis is caused by inhalation of crystalline silica. The term silicosis is derived from the Latin silex, meaning flint. The disease probably existed as long ago as the Paleolithic period, when flint implements were used extensively,342 and the hazards of silica were recognized by the ancient Egyptians, Greeks, and Romans. Pathologic examinations of Egyptian mummies have revealed classical silicotic nodules.
In more recent times the disease has been particularly associated with mining, stone cutting, grinding, and sandblasting. The death rate in some exposed groups has been extraordinary. For example, workers in the cutlery industry in Sheffield, England, in the mid-19th century rarely lived beyond 40 years of age, and many died of silicosis before they reached 30 years of age.342 In the United States, massive loss of life due to acute silicosis was observed in workers at the Hawk’s Nest tunnel in West Virginia in the early 1930s. This incident, described as America’s worst industrial accident, provided the impetus for development of dust control standards.343
In industries where regulations have been applied, for example, the Vermont granite industry, there has been a dramatic decrease in the incidence of silicosis.344,345 Although current regulations appear adequate to protect the vast majority of workers, outbreaks of silicosis similar to those described historically still occur in unregulated or poorly regulated industrial settings,346 and sporadic cases resulting from unusual or avocational sources of exposure are still being reported.345
Mineralogy
Silica (chemical formula SiO2) is a common mineral of the earth’s crust and exists in amorphous and crystalline forms. The latter include quartz, tridymite, cristobalite, stishovite, and coesite, and are formed under conditions of high heat and pressure. The toxicity of silica appears to depend on a tetrahedral crystalline structure; such polymorphs as amorphous silica and stishovite, an octahedral crystalline silica, are relatively nonfibrogenic.347 Most occupational silica exposures are exposures to quartz, but calcining of diatomaceous earth leads to the formation of cristobalite.
Sources of Exposure
Major sources of occupational exposure to crystalline silica are shown in Table 24–22. The number of people currently employed in these industries is not known; however, NIOSH estimated in 1986 that 2.3 million workers are exposed to quartz dust1 and that number was still around 2,024,024 in 1994.348 It is currently estimated that there are approximately 1500 cases of silicosis diagnosed annually in the United States.345 Most cases today occur in unregulated or poorly regulated industrial settings or in situations where the exposure was not recognized.345 A radiographic survey carried out in 1983 showed that typical nodular silicosis occurred at a very low rate (less than 1%) in workers exposed to quartz levels averaging less than the current permissible limit of 100 g/m3.349 More unusual sources of exposure causing silicosis are inhalation of scouring powder,350 inhalation of “crack” cocaine,351 exposure in the electronics industry,352 grinding maize, cooking with biomass fuels in enclosed spaces353 (see Miscellaneous Conditions, later), living in desert-like terrain,354,355 and the cutting of gemstones.356
Clinical and Radiographic Features of Silicosis
Characteristic radiographic appearances of chronic silicosis are small, rounded opacities less than 1 cm in diameter predominating in the upper lung zones. In more advanced cases, the opacities may be disseminated throughout all lung fields. For epidemiologic and legal purposes, they are classified by the system developed by the ILO according to their size, shape, location, and profusion.273 Pathologic studies have shown that exposure to silica-containing dust can result in cryptic pulmonary disease that is not detectible on standard posteroanterior radiographs.357 The nodules of silicosis may calcify and, with increasing degrees of abnormality, tend to coalesce to form large irregular opacities that are usually bilateral and symmetric. Involvement of the hilar lymph nodes by silicotic nodules leads to their enlargement and the development of “eggshell” calcifications, which are considered almost pathognomonic of silicosis.
Acute or accelerated silicosis (silicoproteinosis) may occur within weeks or months of high-level exposure. Patients with acute silicosis may present with severe dyspnea, fatigue, weight loss, and pleuritic pain. Death from this disease usually occurs within 1 to 2 years. The radiographic appearances of silicoproteinosis are those of an alveolar pattern having a ground-glass appearance. Irregular opacities indicative of diffuse interstitial disease are sometimes seen in acute silicosis but may also be associated with the chronic form. Radiographic studies have shown that all forms of silicosis can progress even after cessation of exposure.358,359
In cross-sectional studies, mild forms of chronic silicosis are not usually associated with severe or disabling abnormalities of pulmonary function, whereas more severe forms of silicosis (particularly complicated pneumoconiosis) generally show a restrictive pattern of disease with small noncompliant lungs, arterial hypoxemia, and relatively normal flow rates.345,360 However, as is true of CWP, longitudinal studies have demonstrated that silica exposure is associated with chronic airflow obstruction.126–129,132,133,361,362 The issue of dust-induced airflow obstruction and its anatomic basis was discussed in more detail earlier (see Disease Caused by Inhaled Gases and Fumes). Although epidemiologic studies make clear the role of dust in producing airflow obstruction, in the individual patient it may be difficult to attribute increases in airflow resistance to silica dust if the worker is a cigarette smoker.345
Cases of complicated silicosis (PMF) are associated with a high likelihood of developing respiratory failure with cor pulmonale. Caplan’s syndrome should also be considered in a patient with rapidly developing large nodules on the radiograph who is seropositive for rheumatoid factor.
Silicosis is strongly associated with the development of tuberculosis. With the exception of silicotuberculosis, there is no effective treatment for silicosis, although corticosteroid therapy may have an effect in accelerated silicosis.363,364
Pathogenesis
The toxicity of silica is associated primarily with its crystalline forms. Amorphous silica is relatively nontoxic.365 On an equivalent weight or surface area basis, the toxicity of crystalline silica is much greater than other nonfibrous mineral dusts found in the occupational setting. Investigations into the nature of its toxicity have concentrated on the interactions that occur between the silica particle and cell membranes. Early theories invoking toxicity through the solubility of silica have been discounted. Theories based on hydrogen donation by polymeric silicic acid to form complexes with phospholipids in cell membranes and/or the reaction of silanol (hydroxyl) groups on the particle surface with cell membranes also do not adequately explain the toxicity of silica.366 More recently, free radicals have been implicated in the pathogenesis of silica-induced cell membrane injury. Surface silanol groups (SiOH) complexed with ferric ions are able to generate the potent oxidant, hydroxyl radical, through the Fenton reaction.367 Silicon free radicals are also generated at the surface of the particle when the silica crystal is freshly fractured.368,369 In vitro studies have shown a close correlation between the generation of surface free radicals and cytotoxicity.368 These surface chemical properties change with the age of the surface,370 a factor that may account for differences in toxicity seen in some animal and epidemiologic studies. Other surface properties, such as surface area, charge, and shape, play a role in the toxicity of silica.371
Experiments where the silica particle surface is chemically modified or coated have shown marked reductions in toxicity.371,372 Surface modification experiments have included such compounds as surfactant,373,374 organosilane,375 the polymer polyvinylpyridine-N-oxide (PVPNO),376 tetramethylammonium ions,377 and soluble aluminum.378 In contrast, etching of the surface appears to increase its toxicity.379
Regardless of the mechanism of induction, it is generally agreed that quartz particles are membranolytic and cytotoxic to macrophages and probably to other cells.370 Exposure to quartz results in the release of inflammatory cytokines such as interleukin-6, tumor necrosis factor-, platelet activating factor, and transforming growth factor-.380 The interaction of these cytokines with T lymphocytes and fibroblasts is thought to lead to fibroblast stimulation and collagen deposition,381 eventuating in the silicotic nodule.
The pathogenesis of acute silicosis, which morphologically appears as alveolar proteinosis (lipidosis), probably involves increased surfactant formation and decreased surfactant degradation.382 Genetic polymorphisms in genes controlling cytokine production may also be important in the pathogenesis of silicosis.383,384
Silica exposure is also associated with local and systemic alterations in immune function. These latter effects may account for the association of silicosis with collagen vascular disease, renal abnormalities, rheumatoid pneumoconiosis and susceptibility to infection with Mycobacterium tuberculosis and Mycobacterium avium intracellulare.345,385–391
Acute silicosis (silicoproteinosis) |
Chronic silicosis |
Simple silicosis (nodules) |
Complicated pneumoconiosis (massive fibrosis) |
Rheumatoid pneumoconiosis (Caplan’s syndrome) |
Small airways disease |
Emphysema* |
Interstitial fibrosis |
Lung cancer* |
*Evidence exists both for and against these associations.
Pathologic Features
Chronic Silicosis
Table 24–23 lists the diseases caused by silica. The classical or nodular lesions of silicosis take many years and usually decades to develop. They are characterized by small, rounded, pale, fibrous nodules that are sharply demarcated from the surrounding lung parenchyma (Fig. 24–28). The nodules are more common in the upper and posterior regions of the lung and are often found scattered in the visceral pleura. The latter, referred to as “candle wax” lesions,392 range from 3 to 10 mm in diameter, exhibit a pale waxy dome, and often have a surrounding ring of black pigment (Fig. 24–29). In addition to subpleural sites, silicotic nodules tend to form in regions of the lung with a high density of lymphatic channels; these include the interlobular septa and the peribronchial connective tissues. They are not confined to these sites, however, and may originate in any part of the lung parenchyma, including the alveolar spaces. The pleural and parenchymal nodules are palpable, firm, and frequently show central calcification. Although the centers of the classic lesion are pale, most silicotic nodules become pigmented due to concomitant exposure to carbonaceous or other colored dusts.
Silicotic PMF appears to form through the conglomeration of smaller nodules (Fig. 24–20). The lesion is defined by its maximum diameter. The Silicosis and Silicate Disease Committee of NIOSH defined PMF as a lesion greater than 2 cm in diameter.392 However, it should be borne in mind that the radiologic definition established by the ILO refers to an opacity on the chest x-ray greater than 1 cm in diameter.273 For medicolegal purposes and to establish equivalency with the radiographic definition, it is recommended that the 1 cm size definition of PMF be used for pathologic specimens. In all cases, it is essential to document the exact size of the larger nodular lesions in the lungs of a patient with silicosis.
FIGURE 24–28 Chronic silicosis showing classic features of silicotic nodule. (A) The nodule shows degenerate collagen and amorphous necrosis at center surrounded by concentrically arranged hyalinized collagen fibers. A cellular mantle is seen at periphery. (B) Close-up of periphery showing, at top, hyalinized collagen fibers, and at bottom, histiocytic cells. By polarizing microscopy, many of the cells contained particles consistent with silica.
FIGURE 24–29 Subpleural silicotic nodule. Three discrete silicotic nodules are seen in the subpleural connective tissues. The lesion is capped by a layer of hyalinized collagen. Pigment is seen at the periphery of the nodules.
PMF is usually symmetrically bilateral. PMF lesions may undergo cavitation and are associated with destruction of the lung parenchyma and bronchovascular structures. Marked distortion of the adjacent lung is seen due to fibrotic contraction of the lesion. Mycobacterial infection should be considered in cases showing cavitation, and appropriate attempts should be made to isolate and identify the organisms.
The microscopic appearances of simple silicotic nodules are characteristic and unlikely to be confused with other pathologies. The nodule is composed of concentrically arranged, whorled, bundles of mature hyalinized collagen showing variable calcification within the central region. The nodule itself may be relatively free of pigmentation but is usually surrounded by a more cellular periphery consisting of dust-containing macrophages, fibroblasts, reticulin, and occasional lymphocytes (Fig. 24–28B). Polarizing microscopy usually shows poorly birefringent particles consistent with quartz both within the hyalinized nodule and at the periphery. Brightly birefringent particles and particles with acicular and needle-shaped profiles, indicative of silicates, tend to be concentrated at the periphery.
Patients with accelerated silicosis may show evolving forms of the classical nodules. These nodules are more cellular and have a more granulomatous appearance (Fig. 24–30). They consist of histocytic cells enmeshed in a variable amount of mature and immature collagen and reticulin. Hyalinization and circular orientation of the collagen fibers may be seen in embryonal form.
When the histocytic reaction is florid and the dust inconspicuous, the reaction may simulate a fibrous histiocytoma.393,394
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree