Occupational Lung Disease
INTRODUCTION
• The workplace contains a wide range of materials and conditions that can potentially aggravate pre-existing conditions or cause pulmonary disease in susceptible hosts. Table 26-1 lists a number of some relatively common potentially hazardous agents.
• Diagnosis of workplace-related pulmonary disease requires a high index of suspicion because there may be no clear temporal relationship between an exposure and the subsequent development of signs and symptoms, which may be nonspecific and fleeting.
• Obtaining a detailed occupational history from a patient with a possible workplace-related pulmonary disease is an essential part of the diagnostic evaluation.1
 The occupational history is a comprehensive list of the activities and environments of all remunerative or volunteer work the patient has ever performed, including short-term/temporary/military jobs and hobbies, which is compiled to identify all exposures (Table 26-2).
The occupational history is a comprehensive list of the activities and environments of all remunerative or volunteer work the patient has ever performed, including short-term/temporary/military jobs and hobbies, which is compiled to identify all exposures (Table 26-2).
 Assessment of the home environment, especially during childhood, emphasizing biomass fuel exhaust, radon, and mineral dust exposures may also be important.
Assessment of the home environment, especially during childhood, emphasizing biomass fuel exhaust, radon, and mineral dust exposures may also be important.
• General management principles
 The patient should avoid further exposure to the offending agent. This intervention may involve a change in job responsibilities and patients should be made aware of the fact.
The patient should avoid further exposure to the offending agent. This intervention may involve a change in job responsibilities and patients should be made aware of the fact.
 Supportive care measures which will depend upon individual patient requirements:
Supportive care measures which will depend upon individual patient requirements:
 Supplemental oxygen
Supplemental oxygen
 Pulmonary rehabilitation
Pulmonary rehabilitation
 Tobacco cessation
Tobacco cessation
 Bronchodilators
Bronchodilators
 Influenza/pneumonia vaccinations
Influenza/pneumonia vaccinations
 Because disease can progress even after exposure has ended, serial imaging and pulmonary function tests (PFTs) are recommended in the first years after retirement.
Because disease can progress even after exposure has ended, serial imaging and pulmonary function tests (PFTs) are recommended in the first years after retirement.
• Issues of impairment, disability, and workers’ compensation frequently arise with a diagnosis of workplace-related pulmonary disease.
 Impairment means objectively determined abnormality of functional assessment.
Impairment means objectively determined abnormality of functional assessment.
 Disability implies inability to perform certain tasks owing to impairment.
Disability implies inability to perform certain tasks owing to impairment.
 The disability certification process often involves multiple agencies and procedures that vary from state to state.
The disability certification process often involves multiple agencies and procedures that vary from state to state.
 For assistance with definitions and criteria, the American Medical Association Guides to the Evaluation of Permanent Impairment is a valuable resource.2
For assistance with definitions and criteria, the American Medical Association Guides to the Evaluation of Permanent Impairment is a valuable resource.2
ASBESTOS-ASSOCIATED LUNG DISEASE
General Principles
• Asbestos is composed of hydrated silicates with varying combinations of other elements such as sodium, magnesium, and iron.
• Asbestos can be classified according to the shape of its fibers: amphibolites which are linear fibers or serpentines which are curly fibers.
• Asbestos fibers can damage lung parenchyma and pleura, causing both benign and malignant disease by complex processes that are incompletely understood.3,4
 Fibers can be suspended in air and inhaled.
Fibers can be suspended in air and inhaled.
 Inhaled fibers penetrate deeply into the lungs and cellular structures.
Inhaled fibers penetrate deeply into the lungs and cellular structures.
 Fibers are incompletely cleared.
Fibers are incompletely cleared.
• All asbestos-containing materials, whether made from amosite, crocidolite, tremolite, or chrysotile, can cause fibrosis, lung cancer, and diffuse malignant mesothelioma.
• Clinical and radiographic manifestations of disease may be delayed for decades.
• Asbestos was widely used in construction and manufactured products until 1975. Routes for exposure include:
 The manufacture of asbestos-containing products.
The manufacture of asbestos-containing products.
 Removal of floor tiles, insulated pipes, roofing materials, brake linings, and other asbestos-containing materials currently in place.
Removal of floor tiles, insulated pipes, roofing materials, brake linings, and other asbestos-containing materials currently in place.
 Employment in the construction, maintenance, textile, or roofing industries.
Employment in the construction, maintenance, textile, or roofing industries.
TABLE 26-1 POTENTIALLY HAZARDOUS AGENTS IN THE WORKPLACE
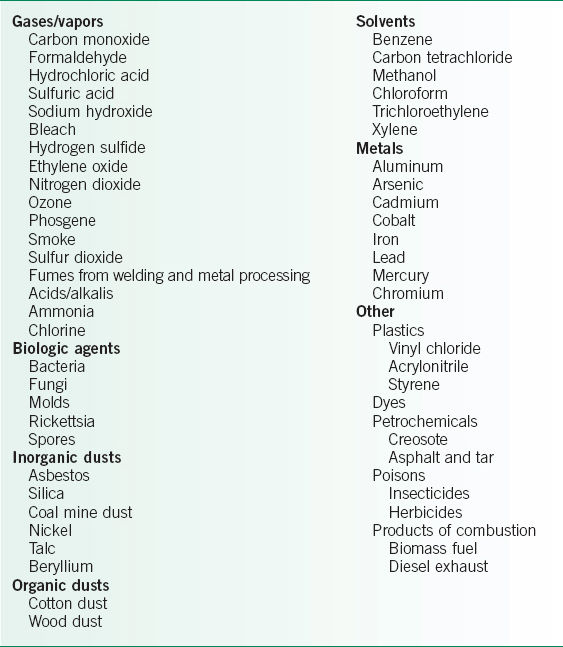
TABLE 26-2 SAMPLE OCCUPATIONAL HISTORY

Diagnosis
• The exposure history may be essential to making the diagnosis. The patient should have a history of exposure to asbestos fibers and a suitable latency period before development of symptoms or radiographic findings.
 Pleural effusions >1 year
Pleural effusions >1 year
 Pleural plaques >10 years
Pleural plaques >10 years
 Asbestosis, lung cancer, diffuse malignant mesothelioma (DMM) >20 years
Asbestosis, lung cancer, diffuse malignant mesothelioma (DMM) >20 years
• The presentation, examination, PFTs, and radiologic findings can be nonspecific.3
 Patients may complain of cough, persistent progressive dyspnea, and sometimes chest discomfort.
Patients may complain of cough, persistent progressive dyspnea, and sometimes chest discomfort.
 Late inspiratory crackles may be heard on auscultation and clubbing may be seen in some cases.
Late inspiratory crackles may be heard on auscultation and clubbing may be seen in some cases.
 PFTs show decreased lung volumes, especially total lung capacity (TLC) and decreased forced vital capacity (FVC), along with decreased diffusing capacity of the lung for carbon monoxide (DLCO).
PFTs show decreased lung volumes, especially total lung capacity (TLC) and decreased forced vital capacity (FVC), along with decreased diffusing capacity of the lung for carbon monoxide (DLCO).
 Impairment of gas exchange is most sensitively determined by arterial blood gas (ABG) analysis conducted at rest and during exercise.
Impairment of gas exchange is most sensitively determined by arterial blood gas (ABG) analysis conducted at rest and during exercise.
 CT is more sensitive than CXR for detecting subtle findings as well as for characterizing pleural processes.5–7
CT is more sensitive than CXR for detecting subtle findings as well as for characterizing pleural processes.5–7
 Special studies such as bronchoalveolar lavage, tissue biopsy, and sputum evaluations may be necessary to find asbestos fibers if exposure requires documentation.
Special studies such as bronchoalveolar lavage, tissue biopsy, and sputum evaluations may be necessary to find asbestos fibers if exposure requires documentation.
• Asbestosis
 The presence of asbestos fibers may result in a persistent inflammatory process culminating in diffuse interstitial fibrosis, with distortion of the lung parenchyma. Diffuse interstitial fibrosis usually develops no sooner than 20 years after the first and heavy exposure.
The presence of asbestos fibers may result in a persistent inflammatory process culminating in diffuse interstitial fibrosis, with distortion of the lung parenchyma. Diffuse interstitial fibrosis usually develops no sooner than 20 years after the first and heavy exposure.
 CT scan shows multiple abnormalities: curving subpleural lines, parenchymal banding, short peripheral lines, and honeycombing in advanced disease.
CT scan shows multiple abnormalities: curving subpleural lines, parenchymal banding, short peripheral lines, and honeycombing in advanced disease.
 Both bilateral pleural plaques and parenchymal processes must be present to make the diagnosis.
Both bilateral pleural plaques and parenchymal processes must be present to make the diagnosis.
 Pleural disease may result from translocation of fibers into the pleural space to stimulate an inflammatory and fibrotic response.
Pleural disease may result from translocation of fibers into the pleural space to stimulate an inflammatory and fibrotic response.
 Pleural thickening
Pleural thickening
 Fibrosis of the visceral pleural with adhesions to the parietal pleura occurs, obliterating the pleural space and extending into lung parenchyma.
Fibrosis of the visceral pleural with adhesions to the parietal pleura occurs, obliterating the pleural space and extending into lung parenchyma.
 CXR shows widely distributed plaques that do not spare the apices or the costophrenic angles.
CXR shows widely distributed plaques that do not spare the apices or the costophrenic angles.
 Plaques are invariably asymptomatic.
Plaques are invariably asymptomatic.
 Rounded atelectasis
Rounded atelectasis
 Pleural thickening may entrap a section of lung, causing atelectasis and associated volume loss.
Pleural thickening may entrap a section of lung, causing atelectasis and associated volume loss.
 CXR shows thickened pleura surrounding a section of atelectatic lung with a so-called comet tail extending in the direction of the hilum.
CXR shows thickened pleura surrounding a section of atelectatic lung with a so-called comet tail extending in the direction of the hilum.
 Pleural effusion
Pleural effusion
 This is the earliest clinical phenomenon, occurring as early as 1 year, but more typically, longer than 10 years after exposure.
This is the earliest clinical phenomenon, occurring as early as 1 year, but more typically, longer than 10 years after exposure.
 Patients may complain of chest pain and breathlessness.
Patients may complain of chest pain and breathlessness.
 CXR usually shows a unilateral effusion but it may be bilateral, either synchronous or metachronous.
CXR usually shows a unilateral effusion but it may be bilateral, either synchronous or metachronous.
 Thoracentesis yields an exudative, sometimes bloody effusion. Fibers are not often found in pleural fluid.
Thoracentesis yields an exudative, sometimes bloody effusion. Fibers are not often found in pleural fluid.
• DMM
 DMM is a malignant process of the parietal surface of the thoracic and/or abdominal cavities that invades heart and lung by direct extension.
DMM is a malignant process of the parietal surface of the thoracic and/or abdominal cavities that invades heart and lung by direct extension.
 Almost all DMM in the United States is due to asbestos exposure. Exposure may have been apparently minimal, indirect, and not occupational. For example, helping a parent to clean work clothes as a child or being present during a ship refitting.
Almost all DMM in the United States is due to asbestos exposure. Exposure may have been apparently minimal, indirect, and not occupational. For example, helping a parent to clean work clothes as a child or being present during a ship refitting.
 Exposure almost always occurred >20 years before clinical manifestations.
Exposure almost always occurred >20 years before clinical manifestations.
 Radiographic findings include lobulated growth over the parietal pleural surface.5
Radiographic findings include lobulated growth over the parietal pleural surface.5
 The diagnosis is usually established by the surgeon’s description and confirmed by tumor biopsy.
The diagnosis is usually established by the surgeon’s description and confirmed by tumor biopsy.
 There is no curative treatment. The prognosis for this malignancy is very grim but new combined surgical and chemotherapeutic regimens show some therapeutic promise.
There is no curative treatment. The prognosis for this malignancy is very grim but new combined surgical and chemotherapeutic regimens show some therapeutic promise.
• There is an association of asbestos-related lung disease and lung cancer.3,4,6–9
 Asbestos has been classified by the International Agency for Research on Cancer (IARC) as group 1, carcinogenic to humans.10 Exposure to asbestos, both in amphibole or serpentine forms, clearly is associated with increased lung cancer risk.
Asbestos has been classified by the International Agency for Research on Cancer (IARC) as group 1, carcinogenic to humans.10 Exposure to asbestos, both in amphibole or serpentine forms, clearly is associated with increased lung cancer risk.
 Tobacco smoking additively, and possibly synergistically, increases lung cancer risk in persons who have even short-term exposure to asbestos. Therefore, tobacco cessation is imperative.
Tobacco smoking additively, and possibly synergistically, increases lung cancer risk in persons who have even short-term exposure to asbestos. Therefore, tobacco cessation is imperative.
 Because asbestos exposure has been associated with a substantial increased risk for lung cancer and early diagnosis may improve outcome, CT surveillance may be employed with expected outcome benefit.
Because asbestos exposure has been associated with a substantial increased risk for lung cancer and early diagnosis may improve outcome, CT surveillance may be employed with expected outcome benefit.
COAL DUST–ASSOCIATED PULMONARY DISEASE
General Principles
• Coal is ranked according to its carbon content, which is determined by the geologic setting in which it was formed.
• Coal dust is primarily carbon but silica, kaolin, mica, metal dusts, and other potentially harmful contaminants may also be present.
• The amount and nature of exposure during coal mining depends upon the rank of coal, quality of dust control measures, and the individual’s work responsibilities.
 Exposure is greatest working underground at the coal face.
Exposure is greatest working underground at the coal face.
 Above-ground workers who operate drills or transport coal may also have sufficient exposure to produce disease in a susceptible host.
Above-ground workers who operate drills or transport coal may also have sufficient exposure to produce disease in a susceptible host.
• The National Institute for Occupational Safety and Health (NIOSH) estimated that 4% of coal workers develop a coal dust-associated pulmonary disease for the period 1995–1999. However, the prevalence increased to 9% during 2005–2006.11
Diagnosis
• The spectrum of clinical manifestations is wide. Patients may be asymptomatic with mild radiographic abnormalities or severely disabled with obvious and advanced radiographic abnormalities.
• Coal workers’ pneumoconiosis
 The hallmark symptom is shortness of breath.
The hallmark symptom is shortness of breath.
 Persistent late inspiratory crackles are heard on examination.
Persistent late inspiratory crackles are heard on examination.
 PFTs may show a restrictive ventilatory defect, with impaired O2 exchange seen first during exercise. Obstructive ventilatory defects are rarely due to coal mine dust and difficult to distinguish from the more common tobacco-associated disease in smoking miners.
PFTs may show a restrictive ventilatory defect, with impaired O2 exchange seen first during exercise. Obstructive ventilatory defects are rarely due to coal mine dust and difficult to distinguish from the more common tobacco-associated disease in smoking miners.
 CXR shows small nodular opacities in the upper lobes in the early stages, which become more numerous and confluent as disease progresses.
CXR shows small nodular opacities in the upper lobes in the early stages, which become more numerous and confluent as disease progresses.
• Progressive massive fibrosis
 Patients complain of shortness of breath and cough.
Patients complain of shortness of breath and cough.
 PFTs may show both obstructive and restrictive ventilatory defects.
PFTs may show both obstructive and restrictive ventilatory defects.
 CXR shows coalescence of nodules >12 mm in size.
CXR shows coalescence of nodules >12 mm in size.
• Chronic obstructive pulmonary disease (COPD) phenotype12,13
 Rarely, never-smoking miners present with cough, expectoration, and/or wheezing with manifestations of airflow obstruction on physical examination and confirmed by PFTs.
Rarely, never-smoking miners present with cough, expectoration, and/or wheezing with manifestations of airflow obstruction on physical examination and confirmed by PFTs.
 CXR is free of interstitial changes.
CXR is free of interstitial changes.
 If no other cause for this clinical presentation is found (e.g., bronchiectasis, asthma, chronic exposure to biomass fuel combustion smoke, cystic fibrosis, α1-antitrypsin deficiency) it should be attributed to coal dust.
If no other cause for this clinical presentation is found (e.g., bronchiectasis, asthma, chronic exposure to biomass fuel combustion smoke, cystic fibrosis, α1-antitrypsin deficiency) it should be attributed to coal dust.
• Industrial bronchitis: This diagnosis is associated with a clinical picture of cough during times of exposure that resolves with cessation of coal mine dust exposure. No other associated impairment is seen.
• There is no specific association between coal mining and lung cancer, though there is some possible uncertainty in this regard.14 As with the general population, when miners are exposed to multiple carcinogens, including radon gas and cigarette smoke, they are at increased risk for lung cancer.
SILICA-ASSOCIATED LUNG DISEASE
General Principles
• Silica (SiO2), in its amorphous form, is noncrystalline and relatively nontoxic if inhaled. In its crystalline form, most commonly occurring as quartz, it clearly can cause pulmonary toxicity if inhaled.15–17
• A detailed occupational history may be necessary to determine all possible routes of silica exposure.
 Found in soil and rock, it is a hazard for tunnelers, sandblasters, millers, and foundry workers.
Found in soil and rock, it is a hazard for tunnelers, sandblasters, millers, and foundry workers.
 It is also found in manufactured materials as diverse as plaster and toothpaste.
It is also found in manufactured materials as diverse as plaster and toothpaste.
• Workers who believe they worked under safe conditions may still have significant potential risk of developing disease regardless of the chronology of exposure.
Diagnosis
• Acute silicosis
 Acute silicosis may develop within weeks to months after exposure to very high concentrations of silica in small particles of airborne dust, such as may occur when sandblasting, rock drilling, tunneling, or quartz milling in an unprotected manner.
Acute silicosis may develop within weeks to months after exposure to very high concentrations of silica in small particles of airborne dust, such as may occur when sandblasting, rock drilling, tunneling, or quartz milling in an unprotected manner.
 Patients develop dyspnea, hypoxemia, and possible respiratory failure, which may be lethal.
Patients develop dyspnea, hypoxemia, and possible respiratory failure, which may be lethal.
 PFTs show restrictive and/or obstructive ventilatory defects, usually with impaired oxygen gas exchange.
PFTs show restrictive and/or obstructive ventilatory defects, usually with impaired oxygen gas exchange.
 Radiographic findings include abundant ground-glass infiltrates seen on both CXR and CT.
Radiographic findings include abundant ground-glass infiltrates seen on both CXR and CT.
 A subset of acute silicosis patients develop silicoproteinosis, which mimics pulmonary alveolar proteinosis radiographically and pathologically.
A subset of acute silicosis patients develop silicoproteinosis, which mimics pulmonary alveolar proteinosis radiographically and pathologically.
• Accelerated silicosis
 Accelerated silicosis develops 2–10 years after heavy exposure.
Accelerated silicosis develops 2–10 years after heavy exposure.
 Patients complain of progressive exertional dyspnea and cough.
Patients complain of progressive exertional dyspnea and cough.
 Patients may have restrictive and/or obstructive ventilatory defects.
Patients may have restrictive and/or obstructive ventilatory defects.
 CXR and CT show multiple small nodules in the upper and midzone regions of the lungs.
CXR and CT show multiple small nodules in the upper and midzone regions of the lungs.
• Chronic silicosis
 Chronic silicosis develops after ≥10 years of exposure to relatively low concentrations of silica.
Chronic silicosis develops after ≥10 years of exposure to relatively low concentrations of silica.
 Patients report progressive exertional dyspnea and cough.
Patients report progressive exertional dyspnea and cough.
 Patients may have restrictive and/or obstructive ventilator defects.
Patients may have restrictive and/or obstructive ventilator defects.
 CXR and CT demonstrate multiple small nodules in the upper and midzone regions of the lungs, becoming larger and more diffusely distributed with disease progression. Characteristic egg shell calcification may outline enlarged hilar and mediastinal lymph nodes.
CXR and CT demonstrate multiple small nodules in the upper and midzone regions of the lungs, becoming larger and more diffusely distributed with disease progression. Characteristic egg shell calcification may outline enlarged hilar and mediastinal lymph nodes.
 Progressive massive fibrosis results from enlargement and confluence of nodules.
Progressive massive fibrosis results from enlargement and confluence of nodules.
 Progression may occur after cessation of exposure.
Progression may occur after cessation of exposure.
• Patients with silicosis are prone to infection with both TB and nontuberculous mycobacteria. Patients who are tuberculin skin test positive should be given lifelong TB prophylaxis.
• The IARC has classified silica as group 1, carcinogenic to humans.18
 This classification has been somewhat controversial decision because not all studies have shown a clear relationship between exposure to silica and the development of cancer. In many studies, smoking history and other confounding factors must be taken into account. Some studies used unvalidated and unreliable death certificates.
This classification has been somewhat controversial decision because not all studies have shown a clear relationship between exposure to silica and the development of cancer. In many studies, smoking history and other confounding factors must be taken into account. Some studies used unvalidated and unreliable death certificates.
 It should be stated, however, that silica exposure, especially with silicosis, might cause a slight increase in the risk for malignancy.19–21
It should be stated, however, that silica exposure, especially with silicosis, might cause a slight increase in the risk for malignancy.19–21
 An official statement of the American Thoracic Society published in 1997 noted that, “the balance of evidence indicates that silicotic patients have increased risk for lung cancer. It is less clear whether silica exposure in the absence of silicosis carries increased risk for lung cancer.”22
An official statement of the American Thoracic Society published in 1997 noted that, “the balance of evidence indicates that silicotic patients have increased risk for lung cancer. It is less clear whether silica exposure in the absence of silicosis carries increased risk for lung cancer.”22
 Until the relationship among silica exposure, silicosis, and the development of lung cancer can be clarified, it seems prudent to recommend that abnormalities seen on CXR should be followed closely, and any findings concerning for malignancy should be evaluated with chest CT and tissue diagnosis as appropriate.
Until the relationship among silica exposure, silicosis, and the development of lung cancer can be clarified, it seems prudent to recommend that abnormalities seen on CXR should be followed closely, and any findings concerning for malignancy should be evaluated with chest CT and tissue diagnosis as appropriate.
WORKPLACE- AND ENVIRONMENT-ASSOCIATED BRONCHIAL REACTIVITY
General Principles
• Occupational asthma is characterized by variable airflow limitation and/or airway hyperresponsiveness attributable to the workplace environment, although the syndrome can develop outside the workplace as well.
• IgE-mediated immunologic mechanisms are not necessarily responsible.
• Individual variability in genetic susceptibility to disease, symptom presentation, and response to therapy, in addition to the differences in apparently similar workplaces produce a very diverse clinical picture.
• The clinician may be required to opine if such a worker has occupational asthma or a pre-existing asthma phenotype aggravated by the workplace.
• In almost all settings, there is substantial individual variation of the dose–response and the type of symptoms that result.
 Several workers may experience apparently similar exposures in an industrial spill but not all are adversely affected.
Several workers may experience apparently similar exposures in an industrial spill but not all are adversely affected.
 Workers with either retrospectively identified or extremely quiescent atopy may have a greater susceptibility to develop latency-associated occupational asthma of any sort (immunologic and nonimmunologic), especially with repeated exposures.23
Workers with either retrospectively identified or extremely quiescent atopy may have a greater susceptibility to develop latency-associated occupational asthma of any sort (immunologic and nonimmunologic), especially with repeated exposures.23
• Nonantigenic chemicals such as hydrochloric acid, sulfuric acid, diacetyl sodium hydroxide, chlorine, other inorganic acids, alkalis, and low–molecular-weight irritants can induce this syndrome either immediately after a single massive exposure, or, more slowly, after multiple, less intense exposures. Chronic exposure to formaldehyde, pesticides, insecticides, solvents, isocyanates (toluene diisocyanate, methylene diphenylisocyante, hexamethylene diisocyanate), and cleaners can produce similar clinical responses.24
• Consideration should also be given to immunologic agents such as cotton, textile dust exposures, animal, insect, or shellfish allergies; western red cedar dust in the lumber industry; wheat or rye dusts in the baking industry; or other food industry exposures to garlic dust, cinnamon, and mushrooms.
• Flour and isocyanates are the most common culprits in the developed world.25
Diagnosis
• The patient complains of some combination of breathlessness, cough, expectoration, wheezing, and chest tightness.
• Persistent bronchial reactivity is manifested over time by different symptom patterns triggered by irritants differing from the initial etiologic agent.
• In general, irritant triggers include extremes of temperature and humidity, ambient tobacco smoke, perfumes, colognes, hairspray, cooking fumes, products of combustion, and cleaning materials.
• The physical examination may be normal; intermittently, wheezing may be heard on auscultation.
• PFTs are often normal at baseline but may demonstrate airflow obstruction with or without improvement after bronchodilator administration.
 Methacholine challenge test is generally considered diagnostic for the presence of bronchial reactivity.26,27
Methacholine challenge test is generally considered diagnostic for the presence of bronchial reactivity.26,27
 Specific inhalation challenge is sometimes necessary.27
Specific inhalation challenge is sometimes necessary.27
 In some cases, airflow limitation may be demonstrated years after exposure.28
In some cases, airflow limitation may be demonstrated years after exposure.28
 If only small airways dominant disease is present, such as occurred among first responders to the World Trade Center disaster, conventional methacholine test may be normal. In such cases, the only measurable abnormality identifiable may be through the use of impedance oscillometry (IOS).29,30
If only small airways dominant disease is present, such as occurred among first responders to the World Trade Center disaster, conventional methacholine test may be normal. In such cases, the only measurable abnormality identifiable may be through the use of impedance oscillometry (IOS).29,30
• CXR is typically normal.
Treatment
• Environmental
 Environmental control is foremost; patients should not return to the workplace without proper respiratory protection, which can be difficult to achieve.27
Environmental control is foremost; patients should not return to the workplace without proper respiratory protection, which can be difficult to achieve.27
 Persons must be fastidious in their avoidance of other non–workplace-associated triggers, both allergic and irritant.
Persons must be fastidious in their avoidance of other non–workplace-associated triggers, both allergic and irritant.
• Pharmacologic
 Treatment with β2 agonists and inhaled corticosteroids should be the first-order approach to blunt the effect of inadvertent breaches in environmental control.
Treatment with β2 agonists and inhaled corticosteroids should be the first-order approach to blunt the effect of inadvertent breaches in environmental control.
 Anticholinergics and systemic steroids seem less successful.
Anticholinergics and systemic steroids seem less successful.
• Reports to patients and third parties
 Because of the nature of reversible or partially reversible airflow obstruction, and because appropriate treatment may preclude an individual’s return to the workplace, the physician may be faced with difficulty in explaining the apparent inconsistency between no measurable impairment on PFTs and the presence of disability owing to dysfunction that develops when returning to the workplace.
Because of the nature of reversible or partially reversible airflow obstruction, and because appropriate treatment may preclude an individual’s return to the workplace, the physician may be faced with difficulty in explaining the apparent inconsistency between no measurable impairment on PFTs and the presence of disability owing to dysfunction that develops when returning to the workplace.
 Although this situation is well understood by the worker, others may be less accepting.
Although this situation is well understood by the worker, others may be less accepting.
• Family and social problems
 Because regularly occurring irritants in the household initiate bronchial narrowing, not only may a former wage earner be unable to return to work, but s/he may also be limited in ability to perform household chores (cooking, cleaning, transport, and shopping).
Because regularly occurring irritants in the household initiate bronchial narrowing, not only may a former wage earner be unable to return to work, but s/he may also be limited in ability to perform household chores (cooking, cleaning, transport, and shopping).
 This limitation may result in unsuccessful role reversal and substantial family stress for which appropriate counseling may be helpful.
This limitation may result in unsuccessful role reversal and substantial family stress for which appropriate counseling may be helpful.
• Special cases
 World Trade Center disaster29–31
World Trade Center disaster29–31
 Many first responders exposed to the mixed dusts and fumes at the site developed persistent and chronic respiratory symptoms.
Many first responders exposed to the mixed dusts and fumes at the site developed persistent and chronic respiratory symptoms.
 Symptoms were triggered by a wide variety of exposures.
Symptoms were triggered by a wide variety of exposures.
 Most had poor response to traditional bronchodilator and anti-inflammatory treatment.
Most had poor response to traditional bronchodilator and anti-inflammatory treatment.
 Spirometry was normal and small airway abnormalities were only found through the use of frequency dependence of compliance and IOS.
Spirometry was normal and small airway abnormalities were only found through the use of frequency dependence of compliance and IOS.
 Popcorn lung32
Popcorn lung32
 In 2002, workers in popcorn factories developed pulmonary symptoms that were sometimes disabling.
In 2002, workers in popcorn factories developed pulmonary symptoms that were sometimes disabling.
 CT images suggested bronchiolitis obliterans.
CT images suggested bronchiolitis obliterans.
 Those who mixed butter flavoring were most frequently affected.
Those who mixed butter flavoring were most frequently affected.
 Flavoring compounds (diacetyl) appeared to have more severe disease developing after months of exposure.
Flavoring compounds (diacetyl) appeared to have more severe disease developing after months of exposure.
 Biomass fuel combustion fumes
Biomass fuel combustion fumes
 Biomass products such as wood, coal, charcoal, or agricultural residue are often used to fuel cook stoves in many parts of the world, including North America.33
Biomass products such as wood, coal, charcoal, or agricultural residue are often used to fuel cook stoves in many parts of the world, including North America.33
 Never-smoking homemakers and children were most frequently exposed.
Never-smoking homemakers and children were most frequently exposed.
 The clinical picture resembled COPD.
The clinical picture resembled COPD.
 When studying COPD epidemiology, biomass fume exposure must be considered as well as genetic propensity, α1-antitrypsin deficiency, and workplace exposures.
When studying COPD epidemiology, biomass fume exposure must be considered as well as genetic propensity, α1-antitrypsin deficiency, and workplace exposures.
HYPERSENSITIVITY PNEUMONITIS
General Principles
• Hypersensitivity pneumonitis (also known as extrinsic allergic alveolitis) develops when susceptible hosts become sensitized and are then repeatedly exposed to any of an enormous number of offending antigens (Table 26-3) that can be found in virtually any environment.
• Although many persons may be exposed to a particular antigen, few develop disease.34,35
• Multiple exposures may be necessary to become sensitized.
• Smokers may be less prone to developing the disease.35,36
• Some attempts have been made to formalize the diagnostic process with major and minor diagnostic criteria but these criteria have not been universally accepted.
Diagnosis
Clinical Presentation
• A high level of clinical suspicion is necessary for diagnosis.
• Patient presentation and radiographic studies can vary according to the stage of the disease.35,37
• Findings are not pathognomonic. Hypersensitivity pneumonitis should be considered when symptoms improve with avoidance of the suspected agent, and recur or worsen with reexposure.34
• Acute phase
 The acute phase resembles an infectious process usually develops between 4 and 12 hours after antigen exposure.
The acute phase resembles an infectious process usually develops between 4 and 12 hours after antigen exposure.
 The patient complains of cough, dyspnea, fever, chills, arthralgia, and malaise.
The patient complains of cough, dyspnea, fever, chills, arthralgia, and malaise.
 The physical examination findings are fever, tachypnea, significant hypoxemia, and respiratory crackles.
The physical examination findings are fever, tachypnea, significant hypoxemia, and respiratory crackles.
• Subacute phase
 This phase develops after continued, prolonged, low-level exposure.
This phase develops after continued, prolonged, low-level exposure.
 Patients report progressive dyspnea, cough, fatigue, anorexia, and weight loss.
Patients report progressive dyspnea, cough, fatigue, anorexia, and weight loss.
 The examination may be normal or may reveal findings such as crackles.
The examination may be normal or may reveal findings such as crackles.
• Chronic phase
 These patients often lack a history of acute episodes.
These patients often lack a history of acute episodes.
 Patients have an insidious onset of cough, progressive dyspnea, fatigue, and weight loss.
Patients have an insidious onset of cough, progressive dyspnea, fatigue, and weight loss.
 The examination frequently reveals basilar crackles.
The examination frequently reveals basilar crackles.
 Up to 50% of patients demonstrate clubbing.
Up to 50% of patients demonstrate clubbing.
• Farmer’s lung
 Disease results from exposure to the fungi Saccharopolyspora rectivirgula (previously known as Micropolyspora faeni) and Thermoactinomyces vulgaris, which are found in moldy hay.36,38
Disease results from exposure to the fungi Saccharopolyspora rectivirgula (previously known as Micropolyspora faeni) and Thermoactinomyces vulgaris, which are found in moldy hay.36,38
 Spores become airborne and are inhaled by susceptible persons.
Spores become airborne and are inhaled by susceptible persons.
 The risk of disease is increased by weather conditions conducive to mold growth, frequent and heavy exposures to hay, and poor-quality ventilation in the workplace.
The risk of disease is increased by weather conditions conducive to mold growth, frequent and heavy exposures to hay, and poor-quality ventilation in the workplace.
• Some very unusual routes of exposure have been reported including a saxophone contaminated with Ulocladium botrytis and Phomo spp.39
TABLE 26-3 HYPERSENSITIVITY PNEUMONITIS—CAUSATIVE AGENTS

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


