CHAPTER 73 Occlusive Disease of the Brachiocephalic Vessels and Surgical Management of Simultaneous Carotid and Coronary Artery Diseases
OCCLUSIVE DISEASE INVOLVING THE BRACHIOCEPHALIC ARTERIES
Pathophysiology
Atherosclerosis of the aortic arch has been recognized recently as a significant contributor to and independent predictor of embolic stroke and generalized atherosclerotic disease, and it is the most common cause of intrathoracic brachiocephalic occlusive disease.1 In addition to its well-known associations with cigarette smoking, peripheral arterial occlusive disease, hypercholesterolemia, hypertension, male sex, and diabetes mellitus, atheroma has also been associated with elevated levels of fibrinogen and homocysteine.2,3 Aortic arch atheroma, first seen early in the patient’s adult life, is characterized by gradually increasing severity.4 The progression of aortic arch atheroma to brachiocephalic occlusive disease is influenced directly by the presence of aggravating risk factors.
Two important pathologic sequelae directly related to aortic arch plaques are atheroembolism and thromboembolism.5 The pathophysiologic mechanisms of this disease involve the formation of atherosclerotic plaques with calcium deposition, thinning of the media, patchy destruction of muscle and elastic fibers, fragmentation of the internal elastic lamina, and thrombi composed of platelets and fibrin. Recent reports indicate that 70% to 100% of all trans-sternally treated occlusive lesions of the great vessels are atherosclerotic in origin.6,7 The significance of atherosclerosis as the predominant cause of brachiocephalic occlusive disease is also highlighted in a report of 5000 patients from the Joint Study of Extracranial Arterial Occlusion, which reported that multiple-vessel occlusive disease involving the brachiocephalic vessels or upper-extremity arterial branches occurred in nearly two thirds of patients.8 Because of the irregular nature of these plaques, embolic phenomena can occur in as many as 30% of these patients. Thrombotic or thromboembolic events predominantly occur in patients with multifocal disease. Embolic phenomena due to ostial disease of the aortic arch branches are uncommon. A cardiogenic source of embolism must be excluded before emboli can be conclusively attributed to arch branch disease.9
Inflammatory disorders, such as Takayasu’s arteritis, giant cell arteritis, temporal arteritis, polymyalgia rheumatica, and radiation-induced arteritis, are among the less common causes of arch branch occlusive disease. The prototypic vasculitis syndrome that commonly leads to occlusive disease of the thoracic aorta and its branches is Takayasu’s arteritis. This disease is characterized by immune-mediated destruction of the medial elastic fibers of the affected vessel, followed by scarring of the media and internal elastic lamina, which causes compensatory intimal proliferation. Takayasu’s arteritis remains an idiopathic large-vessel vasculitis that predominantly affects women of reproductive age. Although it is believed to affect mainly Asians, it has been reported in patients of all ethnicities.10 Cardiac failure and cardiomegaly are usually secondary to hypertension and aortic valvular insufficiency. About 60% of patients with Takayasu’s arteritis require some form of vascular intervention, most commonly involving the coronary arteries, followed by the carotid and upper-extremity arteries.
Clinical Presentation
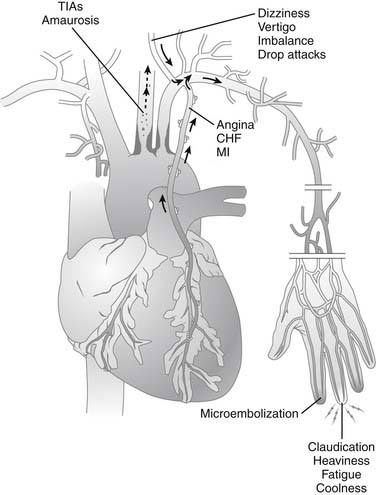
Clinical manifestations of brachiocephalic occlusive disease (Fig. 73-1) are predominately related to the degree of luminal encroachment in the primary vessel affected and the extent of collateral disease if multiple vascular beds are compromised. Stenosis of aortic arch branches can lead to direct ischemia-related consequences or to steal syndromes, in which increases in blood flow to one region directly cause ischemia in another. Involvement of the innominate artery could lead to anterior, posterior, or combined cerebral symptoms, depending on the amount of collateral flow from the contralateral side via the circle of Willis and the extent of concomitant subclavian or common carotid artery (CCA) involvement. Isolated right-sided steal syndromes may occur, but only if the disease arises in the right subclavian artery and the innominate is relatively spared. In contrast, involvement of the left subclavian artery could result in either upper-extremity claudication or vertebral steal manifesting as vertebrobasilar symptoms, depending on the exact location of the stenotic lesion.
Takayasu’s arteritis is associated with a broad spectrum of clinical presentations that range from a fairly indolent chronic course to an acute fulminant disease. The initial symptoms most commonly reported by patients are constitutional and include myalgia, arthralgia, and headaches. Vascular symptoms commonly include claudicatory symptoms, carotidynia, and pulseless extremity. The diffuse involvement of major branches of the aorta contributes to an overall diminution of peripheral pulses in these patients, which is why Takayasu’s arteritis is sometimes referred to as “pulseless disease.” The nonspecific nature of the initial presentation contributes to the delay in the diagnosis of most cases of Takayasu’s arteritis. The Ueno classification system categorizes the disease into four types according to the extent and location of involvement. Types 1 and 3 are characterized by a disease process that affects the aortic arch and its branches.11 Stenosis and occlusion are very typical of Takayasu’s arteritis, and the lesions can be either short and segmental or long and diffuse. De novo aneurysms are rare but have been reported in all major branches of the aortic arch.12,13 Most aneurysms arise from sites of previous anastomoses or surgical repair.14,15
The age at presentation for patients with radiation-induced supra-aortic trunk and upper-extremity disease primarily depends on the age at which they were exposed to radiation, and is often younger than for those with atherosclerotic occlusive disease. They have angiographically atypical lesions that appear diffuse, unlike the focal lesions seen with typical atherosclerosis.16 These patients can present with either embolic or flow-limiting symptoms.
Diagnostic Evaluation
The diagnosis of Takayasu’s arteritis is usually suspected from the patient’s history and clinical presentation and is supported by the findings of specific serologic tests, tests for inflammatory markers, and angiography.17 Angiographic studies show a characteristic pattern of stenosis, poststenotic dilation, aneurysm formation, and occlusion with collateral formation. These findings tend to be localized to the aorta and the proximal aspect of its branches.18,19 Total body arteriography is an important component of the diagnostic workup of these patients to characterize the full extent of the disease and to provide a baseline for future comparison, because these patients require frequent serial imaging and monitoring for the rest of their lives.
Conventional Imaging Modalities
Digital subtraction angiography (DSA)—previously considered the gold standard—offers the opportunity for immediate endovascular intervention if a problem is discovered. As with any intravascular manipulation and imaging technique, the risk for embolic stroke always exists, especially in the presence of atherosclerotic disease and plaques.20 The development of high-resolution computed tomographic angiography with reconstructive capabilities has allowed this modality to substitute for DSA in the assessment of the aortic arch branches in specific circumstances. Computed tomography (CT) and magnetic resonance imaging (MRI) provide valuable imaging for assessing the extent of brachiocephalic disease. Contrast-enhanced MRI with reconstruction provides information equivalent to that obtained from conventional CT angiography. Magnetic resonance angiography (MRA), in addition, can yield useful information about occluded vessels reconstituted via collaterals, because the imaging process is not contrast dependent. Also, MRA provides valuable information about factors that affect the risk for embolization and consequent stroke, including the size, extent, and composition of atherosclerotic lesions. We recommend obtaining a preoperative CT angiogram or MRA for all patients who undergo surgical intervention on the branches of the aortic arch; the images serve as a baseline for future comparisons and for assessing the progression of the disease. Lifelong postoperative surveillance imaging and follow-up are essential components of the care of these patients.21
Ultrasound and Emerging Modalities
Transthoracic echocardiography, although reliable for assessing the ascending aorta, is not ideal for assessing the arch and its branches because of its shallow depth of penetration and because the overlying ribs obscure these vessels. Similarly, transesophageal echocardiography (TEE) is limited in its ability to image the branches of the aortic arch, primarily because of shadowing from the trachea.22 Endovascular ultrasound is an emerging technology that is not widely used at this time.
The advent of transesophageal magnetic coils has made it possible to perform transesophageal MRI (TEMRI).23,24 Although TEMRI allows multiplanar reconstruction and provides better quantification of the extent of aortic atherosclerosis, real-time imaging and assessment of plaque mobility are feasible only with the help of TEE. Nonetheless, TEMRI provides a better assessment of the circumferential extent of atherosclerotic involvement than TEE and could become an important option for imaging the supra-aortic great vessels.
Treatment
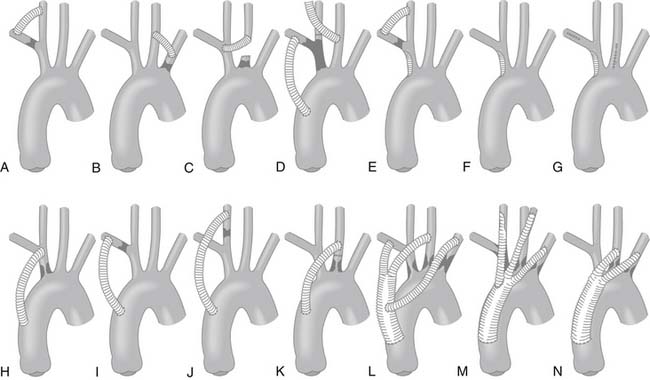
In 1958, DeBakey and colleagues25 reported a large series of cases that included a direct transthoracic repair of the supra-aortic trunks—a major feat at that time. Surgical treatment was further advanced by Crawford and coworkers26 by the use of extra-anatomic bypass techniques, which dramatically decreased the mortality associated with these operations, from 22% to 5.6%. Currently, extra-anatomic bypass with synthetic grafts is the most common technique for treating these complex lesions (Fig. 73-2). The use of shunting for cerebral protection when necessary and the recognition of high-risk patients who are likely to benefit from cerebral protective measures has dramatically curtailed the adverse neurologic consequences associated with these procedures. Alternative techniques include direct endarterectomy, endovascular stenting, and transposition.26 In general, direct surgical approaches, such as bypass techniques, are preferred for multivessel and long-segment disease, whereas endovascular techniques are preferred for isolated ostial disease or short-segment disease. Surgical intervention has reportedly produced survival rates of 98% and relieved symptoms in 94% of patients at a mean follow-up of 7.5 years. Crawford and associates27 reported survival rates of 85% at 5 years, 58% at 10 years, and 25% at 15 years. Hybrid approaches, which combine open and endovascular techniques, have recently been added to the surgeon’s armamentarium for treating supra-aortic occlusive disease safely and expeditiously.
Innominate Artery Occlusive Disease
Innominate artery disease is uncommon. It typically involves the ostium or the proximal aspect of the artery and extends along the posterior and lateral walls. Innominate artery occlusion accounts for only 4.7% of cases of extracranial cerebrovascular disease.28 In these cases, the innominate artery is seldom the only vessel requiring revascularization.29 Reul and colleagues30 found that 60% of patients undergoing revascularization for innominate artery symptoms required intervention in at least one other vessel. Early studies evaluating treatment of innominate atherosclerotic disease favored an extrathoracic approach because of the high morbidity and mortality rates associated with intrathoracic repair.26 Advances in surgical technique and anesthesia, however, produced results equivalent to those of extrathoracic and intrathoracic approaches.31
In patients who present with atheroembolic manifestations, direct operative treatment by excluding the embolic source is an essential element of the treatment strategy for symptom relief. Direct repair or bypass via the transthoracic approach is preferred because it produces less morbidity than using the extrathoracic approach, which is reserved for patients in whom transthoracic repair is contraindicated.32,33 Relative contraindications to intrathoracic revascularization include a heavily diseased or calcified arch, previous thoracic surgery, and advanced age or poor medical condition. Extra-anatomic bypass techniques may be used in cases of primary graft infection, in which one would consider routing the graft well away from the preferred primary route. When revascularization of isolated innominate artery disease is contemplated, two transthoracic options exist: endarterectomy and bypass. Recent advances in endovascular treatment also represent a viable treatment strategy.
Innominate Artery Endarterectomy
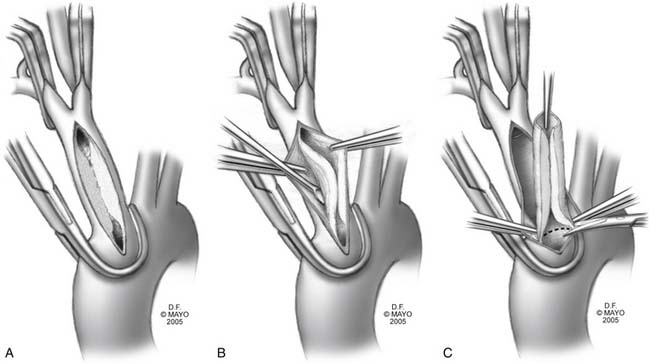
Endarterectomy for isolated branch disease is reported to have excellent results.33 Relative contraindications include inability to clamp the innominate artery, severe arch atherosclerosis, proximal origin of the left CCA (including a common brachiocephalic trunk), transmural arteritis, and multivessel disease; a bypass procedure may be preferable in these cases. Endarterectomy should be avoided in patients with Takayasu’s disease or radiation arteritis, because the transmural inflammatory process complicates the creation of an endarterectomy plane. The endarterectomy proceeds as illustrated in Figure 73-3. Performing intraoperative epiaortic ultrasound before clamping may be of benefit, because most of these patients have aortic arch atherosclerosis. The vessel can be closed primarily, or by patch angioplasty if luminal narrowing is of concern. Long-segment endarterectomies have reportedly been accomplished by extending the arteriotomy or performing separate arteriotomies on the branch vessels.31
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree