DIFFERENTIAL DIAGNOSIS
■ Neurogenic claudication is frequently confused for arterial claudication. Neurogenic claudication is variable—some good days, some bad—whereas arterial claudication is very consistent. Neurogenic claudication can be relieved by the use of spinal support while walking, such as a shopping cart, or wheeled walker. These aids do not influence arterial claudication symptoms.
■ Osteoarthritis of the hips can be frequently confused with arterial claudication. Like arterial claudication, pain is brought on by activity and relieved by rest and is more common with increasing age.
■ Venous claudication from chronic venous outflow obstruction is described as a bursting type pain, comes on at longer distances, requires a longer rest period that requires leg elevation, and is frequently associated with leg swelling and discoloration.
PATIENT HISTORY AND PHYSICAL FINDINGS
■ The diagnosis of aortoiliac occlusive disease can readily be made from a patient’s clinical history and physical examination.
■ Patient’s with aortoiliac disease have up to a 50% risk of concomitant coronary artery disease with the same risk factors of smoking, hypertension, lipid abnormalities, diabetes mellitus, male gender, increased age, and family history.
■ The disease burden in the internal and external iliac blood supply leads to a variety of clinical presentations which is most notable for Leriche syndrome which comprises the symptoms of buttock claudication, impotence in men, muscle atrophy, and absent or diminished femoral pulses.
■ Claudication is the most common presenting symptom and is not limited to the buttocks but can occur in the hip, thigh, and, rarely, in the calf muscles.
■ Impotence as an isolated symptom in men should be evaluated for other possible causes. Impotence is only seen in 30% of men with decreased hypogastric perfusion as there are abundant collaterals from the mesenteric, profunda, and lumbar arteries.
■ Ankle–brachial indices (ABI) are usually diminished but rarely lower than 0.5 in isolated aortoiliac occlusive disease for the same reason cited earlier. It is rare to see critical limb ischemia, which encompasses rest pain and/or tissue loss, in the setting of isolated aortoiliac disease as there are multiple collaterals through the ilioprofunda system.
■ Severe common femoral disease, with both superficial femoral and profunda femoral artery high-grade stenosis or occlusion, can mimic aortoiliac occlusive disease.
IMAGING AND OTHER DIAGNOSTIC STUDIES
■ Ultrasound studies available in noninvasive vascular laboratories can help aid in diagnosis and assess the degree of PAD.
■ ABI measurements can be supplemented by exercise, where a decrease in 15% of the ABI is considered significant as a decrease in peripheral resistance during exercise leads to diminished blood flow distal to the point of stenosis or obstruction.
■ Duplex ultrasound for the aortoiliac system is difficult and is limited by body habitus and bowel gas. With an experienced technician, arterial disease of the intraabdominal vessels can be detected with greater sensitivity.
■ For vascular surgeons, computed tomography angiography (CTA) is the noninvasive imaging study of choice for preoperative planning. Considerations for kidney disease and contrast dye allergies need to be taken into account. The evaluation usually involves the abdomen pelvis and runoff to the feet. It is difficult, especially in tall patients, to evaluate the thoracic aorta during one contrast bolus due to timing of the contrast injection. A CTA study provides accurate estimation of luminal flow and with good visualization of degree of calcification.
■ Magnetic resonance (MR) angiography is also useful but is found to, at times, overestimate the degree of stenosis, and motion artifacts may limit the quality of the study.
■ Direct arteriography under fluoroscopy is considered the gold standard, but improvements in CTA approach this accuracy in evaluating PAD.
■ Arteriography may be difficult in the setting of an iliac occlusion and may need to be performed through the descending thoracic artery using a radial or brachial artery approach.
■ Some surgeons may forego computed tomography (CT) studies and proceed with a contrast digital subtraction angiogram in the setting of a reliable history and physical and/or noninvasive ultrasound testing. This may limit the amount of contrast dye the patient is exposed to by proceeding directly with endovascular intervention. Like CTA, catheter-based arteriographic studies should include infrainguinal outflow of the femoral, popliteal, and tibial vessels, in addition to the abdominal and pelvic views.
■ Although rarely used, pressure catheters can also be used during arteriography to identify significant lesions with a mean arterial pressure drop of 5 to 10 mm Hg across the stenosis considered significant.
■ Preoperative imaging is essential in preoperative planning. The degree of aortoiliac disease in combination with infrainguinal and tibial occlusive disease needs to be considered in choosing the appropriate intervention for the patient.
■ Graft selection, location of cross-clamping, and enlarged collaterals need to be accounted for prior to surgery.
■ Identifying the degree and extent of arterial occlusive disease also enlightens the decision between an open or endovascular approach.
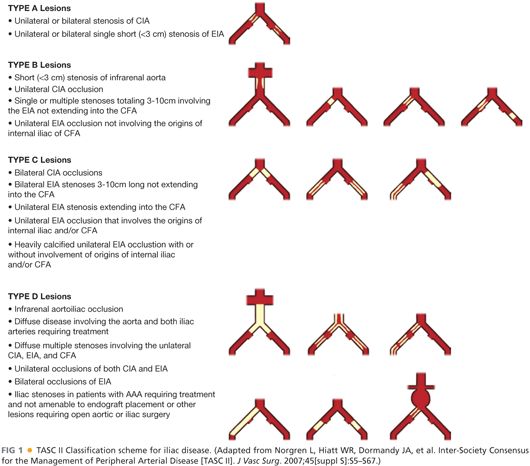
■ The Trans-Atlantic Inter-Society Consensus (TASC) Classification (FIG 1) is a multispecialty consensus approach to managing aortoiliac occlusive disease. Routinely, TASC A and B lesions are treated with endovascular approaches with balloon angioplasty and/or stenting. TASC C and D lesions have a better outcome with an open approach.
SURGICAL MANAGEMENT
■ Before surgical management is pursued, medical management should be initiated due to the high incidence of coronary artery disease with peripheral artery disease.
■ Patients should be advised to quit smoking; placed on a regular walking program, ideally supervised; and started on statin therapy and aspirin when appropriate and tolerated.
■ Preoperative anesthesia visits should include inquiries into cardiac, lung, and renal systems to evaluate overall operative risk.
■ Age and comorbidities should be factored into decisions regarding an open versus endovascular approach, as should procedural durability and invasiveness of the intervention.
■ The aims of therapy in aortoiliac occlusive disease are to relieve symptoms and, in cases of critical limb ischemia, prevent limb loss. Revascularization of the aortoiliac system can be done in a variety of endovascular and open approaches. The choice of procedure depends on the disease pattern, patient risk factors, available resources, and surgeon experience.
■ We describe three common operative interventions for inflow disease: aortobifemoral bypass, femoral–femoral bypass, and femoral endarterectomy.
TECHNIQUES
ISOLATED FEMORAL RECONSTRUCTION FOR LIMB SALVAGE WITH POSSIBLE ENDOVASCULAR INTERVENTION
First Step—Landmarks
■ The patient is placed prone on the operating table, with a soft bump under the knee and the leg slightly abducted and externally rotated. The inguinal ligament marks the transition point from the external iliac artery to the common femoral artery and is identified by connecting the anterior superior iliac spine to the pubic symphysis. The common femoral artery is usually located over the medial third of the femoral head but may be difficult to palpate in aortoiliac occlusive disease. Pre- or intraoperative ultrasound can be used to identify the common femoral as well as the bifurcation into the superficial and profunda arteries.
Second Step—Incision and Exposure
■ Two types of incisions can be made to expose the common femoral artery. A vertical incision above the common femoral artery and bifurcation allows for greater access to the proximal and distal vessels in extensive disease. An oblique incision is better for cosmesis and is made cephalad to the groin crease and is acceptable in focal isolated lesions and in obese patients.
■ After a skin incision is made, the subcutaneous tissue is then dissected with electrocautery with careful attention to ligate superficial crossing vessels to control bleeding and ligating lymphatics to prevent seroma formation. A self-retaining retractor is used to help expose the tissues and is repositioned as the dissection proceeds deeper. Once the femoral sheath is identified and entered the femoral artery lies lateral to the femoral vein. The distal external iliac artery, the common femoral artery, the proximal profunda femoral and superficial femoral arteries are circumferentially dissected with scissors and tagged with a vessel loop or moist umbilical tape using a right angle. Other arterial side branches may be identified and are controlled with vessel loops or temporary clips but rarely ligated in occlusive disease.
Third Step—Clamping
■ Before clamping of the arteries, heparin is given intravenously as a weight-based bolus at 100 units/kg and allowed to circulate for 3 minutes. Heparin has a half-life of approximately 90 minutes and is checked periodically with activated clotting time (ACT) measurements. A range of 250 to 350 is desirable and 1,000 to 3,000 units boluses can be given periodically to maintain this level of anticoagulation. Gaining proximal and distal control of the femoral arteries can be done with a variety of clamps. An angled Novare clamp or femoral C-clamp can be used at the proximal common femoral artery. Next, a profunda clamp is used to clamp the profunda femoral artery and the superficial femoral artery. Clamps are placed on soft areas of the artery past the area of the high plaque burden. Side branches can be controlled with vessel loops pulled tightly around the artery and clamped with a hemostat or temporarily clamped with a medium or large clip.
Fourth Step—Endarterectomy, Tacking sutures
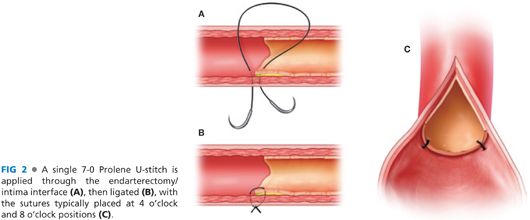
■ A longitudinal incision is made along the exposed common femoral artery using a no. 11 blade scalpel followed by Potts scissors. The adventitia is grasped with vascular forceps and a freer elevator is used to dissect the plaque away from the adventitia. The plaque is removed with blunt dissection unless sharp dissection is needed to dissect the plaque off the posterior wall. Tacking sutures with 7-0 Prolene are used to secure the remaining plaque to the posterior wall to avoid a dissection plane and emboli (FIG 2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


