■ Once the degree and physiologic impact of the disease are determined by noninvasive testing, high-resolution anatomic imaging via either computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) should be obtained for surgical planning.
■ CTAs are currently the gold standard for preoperative planning. They have the advantage of providing information regarding the degree and location of stenosis as well as the anatomy of the arterial wall (including degree of calcification and presence of aneurysms). Three-dimensional reformatting can provide additional valuable information (FIG 2). CTAs, however, are limited by the fact that they involve the use of contrast as well as radiation exposure. MRAs avoid radiation exposure and contrast often, however, at the risk of reduced anatomic precision. Gadolinium magnetic resonance (MR) contrast also entails risk of long-term renal dysfunction.

■ Catheter-based diagnostic aortography also provides anatomic data; however, this study has a number of limitations including the fact that it is an invasive procedure with potential complications. In addition, arteriograms only provide an understanding of the luminal anatomy, occasionally obscuring features such as aneurysms, inclusion cysts, or periarterial inflammation. Particularly for aorto-iliac-femoral disease, preprocedural CTA has the ability to identify significant common femoral disease that may benefit from concomitant open endarterectomy at the time of catheter-based intervention. Alternatively, relying on catheter-based arteriography as the primary diagnostic modality may reduce overall contrast burden, radiation exposure, and need for additional procedures if common femoral level intervention is not required. In general, careful preprocedural physical examination and duplex imaging may suffice to help determine whether the additional cost, risk, and inconvenience of CTA are justified prior to catheter-based intervention for aortoiliac arterial occlusive disease.
SURGICAL MANAGEMENT
■ As with all patients with PAD, initial treatment approach should include comprehensive assessment and management of concomitant cardiovascular disease risk factors. Details regarding maximal medical management of PAD are beyond the scope or purpose of this chapter; at a minimum, however, consideration should be given to beginning statin and antiplatelet therapy prior to intervention, along with consideration of beta blockade and angiotensin receptor blocker or converting enzyme inhibitor therapy in selected patients.
■ Regardless of medical or anesthetic risk, however, all patients with critical limb ischemia should be considered candidates for revascularization when limb loss is a distinct possibility. Despite platitudes to the contrary, major limb amputation above or below the knee is not necessarily a “safer” surgical alternative to multilevel hybrid revascularization. Indications for intervention for intermittent claudication are somewhat more complicated, however. The risks of a procedure are weighed against the potential gain; typically, only patients with severe lifestyle-limiting claudication who have failed nonoperative strategies are offered surgical revascularization.
Preoperative Planning
■ Determining the anatomic distribution of disease is essential to obtaining optimal results. The imperative for precision imaging cannot be emphasized enough—if you cannot appreciate the full extent of disease, you cannot expect to comprehensively address it. As in all aspects of vascular surgery, the biggest disappointments, both during and after the procedure, usually arise from underestimating the extent of underlying disease.
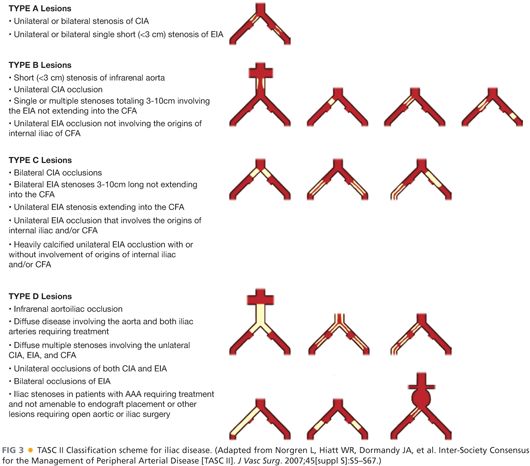
■ The Trans-Atlantic Inter-Society Consensus (TASC) II guidelines provide a classification scheme based on anatomic patterns of disease (FIG 3).2 The recommendations of the TASC II guidelines is an endovascular management for TASC A and B iliac lesions, whereas open surgical reconstruction is recommended for TASC C and D lesions in good-risk patients. Frequently, however, patients with multilevel disease as seen in TASC C and D lesions have more virulent atherosclerotic processes that often make them poorer surgical candidates. In addition, the development of an increasingly sophisticated armamentarium of endovascular tools and strategies are leading more and more vascular surgeons to attempt endovascular revascularization, even for patients with TASC C or D lesions. Further updates of the TASC classification guidelines are under review and will likely be published in the near future, highlighting the dynamic nature of surgical management of this challenging condition.
■ Targeted perioperative risk assessment should be undertaken in appropriate patients, particularly those with reduced exercise tolerance, known or suspected congestive heart failure, clinically significant pulmonary disease, exercise-induced angina, arrhythmias, or those with recent history of myocardial infarction. The presence of additional relevant comorbidities, including diabetes, reduced glomerular filtration rate, iodinated contrast allergies, thrombophilia or coagulopathic disorders, concomitant bacterial infection, or liver disease should also be identified and, when present, evaluated.
Positioning
■ Patients are generally placed in the supine position, either in a hybrid operating suite with fixed imaging capabilities or on a radiolucent table with a mobile imaging unit (C-arm) in a traditional operating room environment.
■ Positioning should be arranged in such as way as to ensure adequate exposure of the entire aortoiliac and femoral vasculature, with room on either side of the patient to rotate the imaging unit to various angles in order to obtain appropriate oblique images. In angiographic parlance, in many important circumstances (such as identifying and protecting the origin of the ipsilateral internal iliac artery), “one view is no view.”
TECHNIQUES
FEMORAL ENDARTERECTOMY
First Step
■ For extended femoral endarterectomy (often requiring exposure of the proximal deep femoral artery as well as the entire length of common femoral artery), optimal exposure is obtained via a longitudinal incision placed directly over the femoral artery (FIG 4). The inguinal ligament should be identified by palpation of the pubic tubercle and anterior superior iliac spine (an oblique line between these two structures is the typical course of the inguinal ligament) and used as a guide for femoral localization. Typically, the femoral artery is located approximately one-third the distance from the pubic tubercle to anterior superior iliac crest. Even when no pulse is palpable, a firm calcified linear mass can usually be palpated in this area. Alternatively, duplex ultrasound or fluoroscopic imaging may be used to ensure accurate placement of the incision. Failure to incise directly over the common femoral artery may increase risk for chronic lymphatic drainage, delayed or complicated wound healing, and femoral nerve or venous injury. Although oblique femoral incisions have gained in popularity, especially when used to obtain femoral access for proximal aneurysm repair, these often do not provide exposure sufficient for comprehensive endarterectomy as previously detailed.
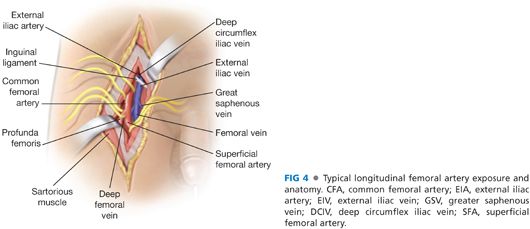
■ The subcutaneous tissues are divided, ligating any lymphatic channels that are encountered. The inferior edge of the inguinal ligament is identified and the common femoral artery is exposed through the femoral sheath as it exits underneath the inguinal ligament.
Second Step
■ Full circumferential dissection of the distal external iliac artery (under the inguinal ligament), the common femoral artery, the superficial femoral artery, and the origin of the deep femoral artery and its initial branches are obtained sequentially (FIG 4).
■ The individual arteries should be assessed for areas of calcification and extensive plaque burden. Soft sections with minimal calcification, or plaque limited to the posterior arterial wall, should be identified for consideration of clamp placement as appropriate for the planned procedure.
■ The inguinal ligament may be divided for adequate exposure of the distal external iliac artery when necessary to ensure adequate endarterectomy When considering the relative margin of distal endarterectomy versus proximal stent placement, it is important to avoid stent placement across the inguinal ligament, as this may greatly reduce long-term patency of the procedure as well as complicate stent delivery through an ipsilateral retrograde sheath. In general, operators should err of the side of more extensive proximal endarterectomies as opposed to distal extension of external iliac stents.
■ Careful ligation of the circumflex iliac vein as it crosses over the external iliac artery under the inguinal ligament should be considered to prevent accidental tearing of the vessel during clamping.
■ External iliac collaterals, like the epigastric artery or circumflex iliac artery, should be preserved during dissection and endarterectomy whenever possible to ensure optimal long-term outcome.
Third Step
■ Once exposure is complete, the common femoral artery can be punctured under direct vision with advancement of a wire under fluoroscopic guidance across the iliac lesion (FIG 5).

■ This eliminates the possibility of creating a retrograde dissection when a wire is passed after the endarterectomy is performed, as well as the need to puncture the endarterectomy patch to gain access.
■ If the disease burden is confined to the common iliac artery or only the proximal external iliac artery, then iliac stenting can proceed at this point, prior to proceeding with the endarterectomy. Occasionally, however, the amount of femoral disease burden is so great that the sheath will be occlusive or otherwise impair runoff, which may limit the ability to obtain digital subtraction angiography (DSA) images during or after stent placement. So consideration should be given to initial endarterectomy depending on individual anatomic circumstances.
■ When retrograde wire passage is not possible due to extensive proximal plaque burden, tortuosity, or other anatomic considerations, antegrade passage from the contralateral iliofemoral system (obtained via either percutaneous or open femoral access) or left axillary or brachial access may be attempted. Obviously, longer sheath/catheter/guidewire combinations will be needed for these procedures and positioning considerations will be affected as well (e.g., arm will need to be exposed and prepped on a radiolucent surface). Once antegrade wire is accomplished, this may be used to deliver treatment devices directly or snared and externalized through the ipsilateral femoral access for retrograde intervention as originally planned.
Fourth Step
■ Leaving the wire in place, systemic anticoagulation is accomplished with sufficient doses of unfractionated intravenous heparin administration and proximal and distal femoral control is obtained with vascular clamps. Especially proximally, a padded clamp should be chosen to allow the external iliac artery to be clamped over the existing wire to prevent or minimize wire-related injury.
■ A longitudinal common femoral arteriotomy is performed to expose the full extent of femoral disease that needs to be addressed to ensure adequate runoff from the iliac intervention. This can almost always be accomplished within the femoral incision itself without need for additional distal femoral bypass procedures, unless extensive forefoot gangrene is present as a consequence of multilevel arterial occlusive disease. Extended deep femoral endarterectomy is highly effective in achieving suitable runoff when few other revascularization options may be available (FIG 4).
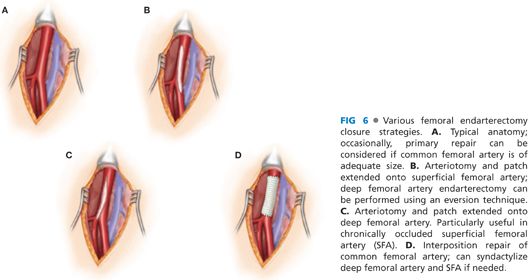
■ The arteriotomy can extend onto either the superficial or deep femoral artery. Occasionally, an eversion endarterectomy of the deep femoral artery can be performed when the arteriotomy extends onto the superficial femoral artery. Alternatively, the arteriotomy may be extended down the deep femoral artery when the superficial femoral artery is chronically occluded. Selection of the reconstruction technique is influenced by the occlusive pathology, level of debility, indications for revascularization, and optimal revascularization strategy (FIG 6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


