Occluded Internal Carotid Artery with Symptoms
CHRISTOPHER ROARK and B. GREGORY THOMPSON
Presentation
A 45-year-old right-handed female mechanic is referred to your office because she has been falling at work. The patient states that these episodes began 6 months ago and have been slowly increasing in frequency, now occurring three to four times per week. She typically notices that after lying under a car in shop she will “abruptly fall down” if she gets up too fast and starts walking quickly. She recovers nearly immediately after falling to the ground. There have been no witnessed episodes of shaking, loss of consciousness, urinary incontinence, or tongue lacerations. This began in the early spring and has progressively worsened through the summer months. She initially felt it was related to dehydration, and she notes fewer “spells” on the days when her water consumption is elevated. She does not regularly go to the doctor and takes no medications. On physical examination, she is neurologically intact. She hand-carries a 24-hour EEG report that is read as normal.
Differential Diagnosis
The patient is describing “drop attacks.” These are characterized by sudden falls without loss of consciousness or warning signs. Key differentials for drop attacks are seizure, cardiac, and thromboembolic causes. Seizures or cardiac events causing these symptoms would often lead to loss of consciousness and would be associated with other neurologic or physical symptoms. Seizures are often—but not always—followed by variable periods of time where the patient does not feel at his or her baseline. This patient is immediately “back to normal.” Thromboembolic phenomena, although common, typically result in an isolated neurologic deficit (e.g., arm weakness only, aphasia only, visual complaints only) during a particular episode. This patient does not describe any focal neurologic deficits and has a normal neurologic exam. The association with postural changes and hydration status could certainly point to a primary cardiac cause of these events and warrants further investigation.
Workup
Holter Monitor
A Holter monitor is ordered, and the patient wears this for 1 week. During this time, she is released back to work and has another attack. There are no arrhythmias noted that would expect to be causative.
MRI/MRA
An MRI/MRA of the brain is performed to look for mass lesion, hydrocephalus, stroke, or vascular abnormality.
Report: No mass lesions, hydrocephalus, or large territory infarcts are noted. The left internal carotid artery (ICA) appears to drop out near its terminus. This could represent severe stenosis or occlusion. There are multiple dilated collaterals at the base of the brain. The right ICA is smaller in caliber at its terminus than would be expected.
Diagnostic Cerebral Angiography
A formal cerebral angiogram is the definitive test for evaluating the cerebral vasculature (both intra- and extracranial).
Report: Rapid, severe tapering of the left ICA at its terminus with some stenosis of the proximal anterior and middle cerebral arteries (Fig. 1). Moderate tapering of the distal ICA on the right is seen. Small collateral vessels are seen at the base of the brain (“puff of smoke”). External carotid artery runs demonstrate a widely patent superficial temporal artery (STA) without evidence of stenosis or vasculitis.
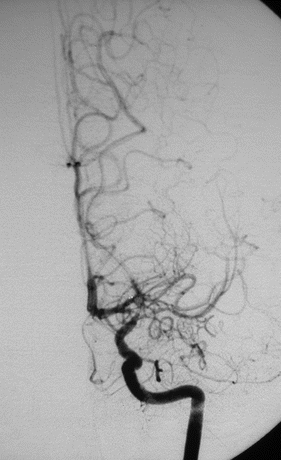
Figure 1 AP angiography with left ICA injection demonstrating rapid tapering of ICA terminus with numerous dilated collateral vessels at the base of the brain.
Xenon CT Scan
To assess the degree and limits of the patient’s collateral circulation, tests that can now be performed include positron emission tomographic (PET) scan, magnetic resonance imaging (MRI) scan with perfusion/diffusion, computed tomographic (CT) scan with perfusion, or xenon CT scanning. In this case, xenon CT scan is ordered.
Report: Xenon CT scan reveals decreased baseline cerebral blood flow in the left ICA distribution (Fig. 2).
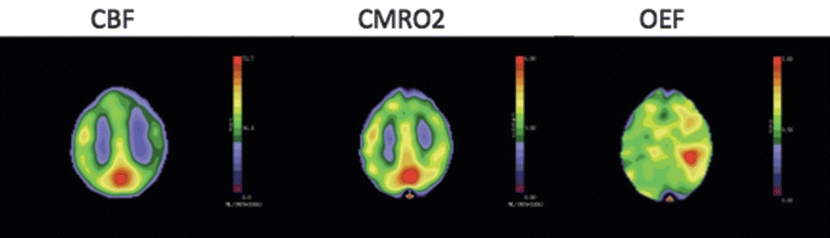
Figure 2 Severe reductions in perfusion pressure below the autoregulatory limit produce a decline in regional cerebral blood flow (CBF) greater than in cerebral oxygen metabolism (CMRO2), thus causing an increase in regional oxygen extraction fraction (OEF).
Diagnosis and Treatment
The angiogram solidifies the diagnosis of moyamoya disease and demonstrates collateral circulation to the frontal, temporal, and parietal lobes. The xenon CT scan confirms the presence of diminished perfusion in the left hemisphere. While surgery for isolated atherosclerotic carotid occlusion has fallen out of favor after the results of two randomized controlled trials (RCTs) demonstrated no benefit, surgical revascularization as a primary treatment of moyamoya disease (a cerebral proliferative angiopathy) is still understood to be of benefit.
This patient presented with symptoms of hemispheric hypoperfusion. Medical therapy relies on antiplatelet agents to minimize thromboembolic complications, of which this patient has had none. Given her young age, symptomatology, and line of work, she would be expected to derive significant benefit from cerebral revascularization.
Surgical Approach
Surgical Decision Making
The results of the EC-IC bypass trial published in 1985 resulted in a significant decrease in the number of these procedures being performed. Significant concerns were raised regarding the large number of patients operated on outside of the trial and the lack of hemodynamic evaluation in the selection of patients. The Carotid Occlusion Surgery Study (COSS) trial was designed to address the hemodynamic risk associated with stroke in the setting of ICA occlusion. The COSS trial was stopped for futility reasons after a planned interim analysis demonstrated no benefit for the surgical arm in the primary outcome ([1] all stroke and death from 30 days after surgery and [2] ipsilateral ischemic stroke within 2 years of randomization). EC-IC bypass volume has subsequently continued to decline. Today, EC-IC bypass is largely reserved for patients with moyamoya disease who have continued to have ischemic strokes despite maximal medical therapy (usually antiplatelet agents such as aspirin). Surgical treatment of moyamoya disease (via direct EC-IC bypass or indirect methods) has been shown to decrease the rate of symptomatic progression to 2.6%.
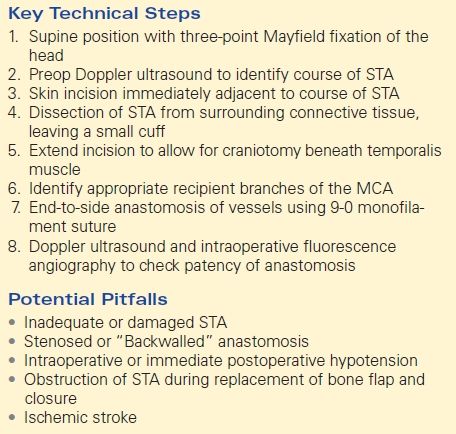
Key Technical Steps
The patient is placed in a Mayfield head-holder to provide three-point fixation. While the patient is in the supine position, the head is turned to the right. A Doppler probe is then used to demarcate the branches of the STA in the scalp. After identification of a suitable donor vessel, the head is shaved and prepped in a sterile fashion. A skin incision is made next to the donor vessel so that it can be identified clearly and dissected free from the surrounding connective tissue. The incision is lengthened so that a small craniotomy may be performed beneath the temporalis muscle. A distal branch of the middle cerebral artery (MCA) is identified on the cortical surface as the recipient vessel. The donor and recipient vessels are joined in an end-to-side anastomosis with an 9-0 monofilament suture. A handheld Doppler probe is used to ensure adequate flow across the anastomosis. When a suitable STA cannot be found, saphenous vein or radial artery can serve as a conduit between the EC and IC circulation.
Potential Pitfalls
EC-IC bypass is dependent on the presence of adequately sized vessels to allow for direct anastomosis. In an adult, the STA usually will have a similar caliber to a cortical MCA branch. Children may not have requisite vessel size to accept and bypass and this contributes to the preference for indirect bypass techniques often used in pediatric moyamoya cases. Occasionally, a surgeon will encounter an inadequate or damaged STA. The preoperative angiogram must be carefully studied to assess the caliber of the STA and whether use of the frontal or parietal branch is preferable. Damage to the STA during the initial opening can also preclude a direct STA-MCA bypass. In cases where an adequate STA is not present, one can use the saphenous vein or radial artery as an interposition graft.
Obstruction of STA during replacement of bone flap and closure can ruin an otherwise technically successful operation. It is imperative to evaluate, and modify if necessary, the bone flap to accommodate a traversing STA. The superficial temporalis fascia near the anastomosed STA must also not be closed tightly such that flow is impeded.
Ischemic stroke is a known risk of any EC-IC bypass procedure. These procedures should be performed at centers with an acceptably low rate of ischemic complication, given that this is the outcome surgery is designed to avoid (Table 1).
TABLE 1. Occluded Internal Carotid Artery with Symptoms