CHAPTER 54 Nuclear Cardiology and Positron Emission Tomography in the Assessment of Patients with Cardiovascular Disease
Nuclear cardiology involves the imaging of the distribution of cardiac radiopharmaceutical agents to characterize physiologic and pathophysiologic processes in the heart. The ability to image myocardial perfusion function and metabolism noninvasively with nuclear techniques has led to the development of a field that has been validated extensively and provides powerful diagnostic and prognostic information in the management of patients with known or suspected coronary artery disease (CAD). Moreover, nuclear cardiology procedures have been employed widely in the evaluation of patients before cardiac and noncardiac surgery. This chapter provides an overview of the concepts and techniques used in nuclear cardiology, and it summarizes its role in the assessment of patients with stable coronary disease or acute coronary syndromes, and in the determination of myocardial viability in patients considered for revascularization. As a complete discussion of the technical and procedural aspects of nuclear cardiology is beyond the scope of this chapter, the central focus will be on the use of nuclear cardiology in patients with known or suspected CAD, with an emphasis on patients undergoing cardiac surgery. For a more detailed discussion, the reader is referred to other, more comprehensive reviews.1,2
General Principles
Nuclear cardiology has been dominated by radionuclide myocardial perfusion imaging (MPI) as a means to evaluate patients with known or suspected coronary disease. MPI is performed to evaluate diagnostically patients with symptoms suggestive of myocardial ischemia, and to risk-stratify patients with known CAD. Radionuclide MPI plays a central role in the evaluation of the cardiac patient and is in widespread clinical use, with more than 7 million MPI procedures performed in the United States annually.3
Equipment and Procedures
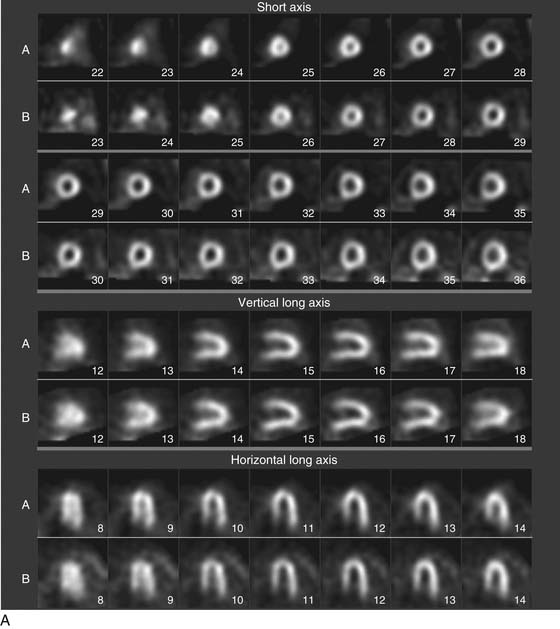
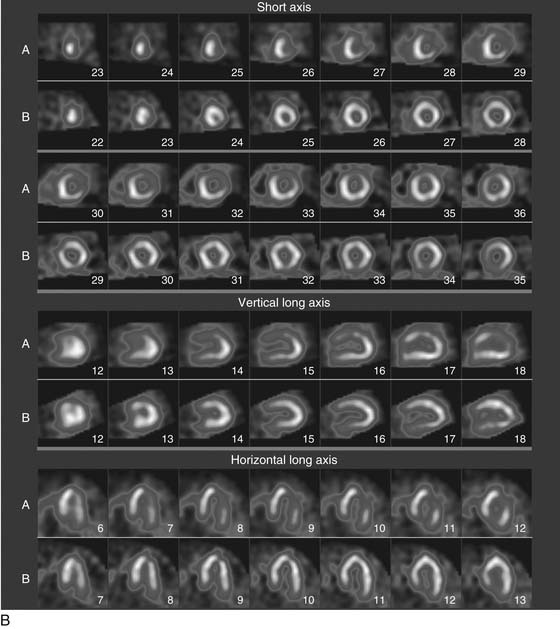
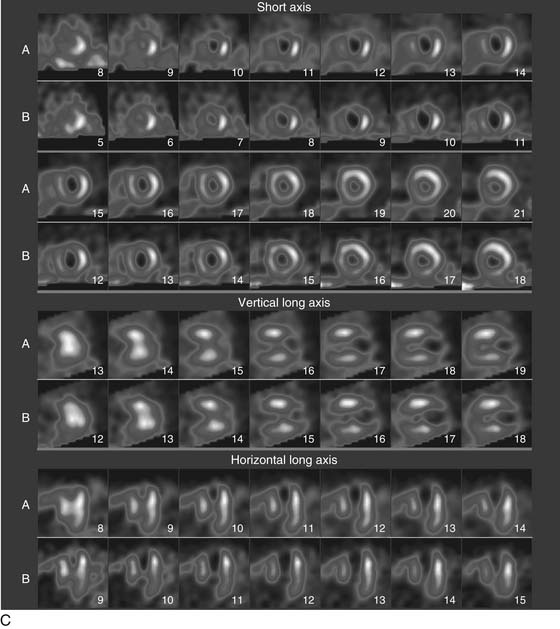
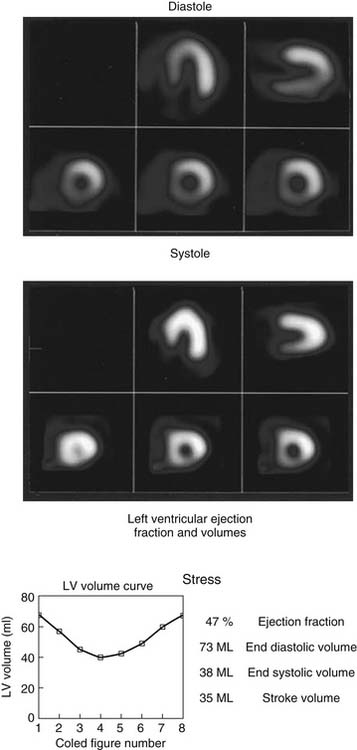
As the camera detector moves around the patient, emitted photons interact with the camera’s crystal and produce scintillations of light that represent the spatial distribution of radioactivity in the patient. The emitted “counts” localized to each region of the myocardium are then stored digitally. This three-dimensional representation of the myocardial perfusion is then processed and reconstructed using computer algorithms to display the acquired information in a series of slices, oriented in the short axis and in the horizontal and vertical long axes of the left ventricle. The slices are then displayed on a computer screen for visual inspection of regional myocardial perfusion as well as computer quantification of tracer uptake. The stress images, acquired after injection of radiotracer during exercise or pharmacologic stress (described later in detail) are displayed adjacent to images acquired at rest, permitting direct comparison of perfusion in the two imaging states. The maximal uptake of radiotracer in the heart is used to represent normal perfusion, and the rest of the counts are considered as relative uptakes to this maximum. As shown in 54-1, a regional perfusion defect on the stress images that is not present on the resting study is considered to be “reversible” and consistent with stress-induced ischemia in that vascular territory. A defect that persists on stress and rest is deemed “fixed” and representative of scar or prior myocardial infarction. Quantitative programs are commonly used to assess the extent and severity of each defect, and this information is then incorporated into the final interpretation of regional myocardial perfusion. The perfusion images are also obtained in concert with electrocardiographic (ECG) gating, which stores the counts with respect to the timing of the cardiac cycle. By gating the acquisition to the R-R interval of the ECG, images from each frame can be summed and displayed as a cine movie of the left ventricle contracting from diastole to systole. Performing gated SPECT facilitates measurement of left ventricular (LV) ejection fraction (LVEF) and LV volumes by automated computer algorithms, as well as by visual inspection of the movie for regional wall motion and thickening (Fig. 54-2).4
A typical SPECT MPI protocol involves the performance of exercise, typically on a treadmill or bicycle, or alternatively a pharmacologic stressor, such as adenosine, dipyridamole, regadenoson, or dobutamine, for patients unable to perform physical exercise. The radiotracer is injected at peak stress and the stressor is then continued for an additional 1 to 2 minutes to maximize myocardial extraction of the circulating tracer. Imaging is performed 30 to 60 minutes later for sestamibi or tetrofosmin, as these agents have only minimal redistribution or washout from the myocardium. The initial imaging can be either at rest or at stress, and both studies can be performed on the same day depending on the patient’s weight. Although 201Tl is used less commonly for stress MPI, it is commonly used for rest imaging in a dual-isotope protocol. In this protocol, 201Tl is injected for resting imaging, followed immediately by stress perfusion imaging with a 99mTc agent, thus obviating the need to wait 2 to 3 hours for the first dose to decay.5 Thus, imaging time is reduced and patient throughput is increased. Recent advances in camera technology and processing have facilitated faster image acquisition and the use of lower dosages of radiopharmaceuticals.
STABLE CORONARY ARTERY DISEASE
Stress Testing in the Detection of CAD
Exercise Stress Testing
Exercise stress testing without adjunct noninvasive imaging is frequently used as the initial screening test in patients without known coronary artery disease who present with chest pain syndromes or symptoms suggestive of ischemia. The premise of exercise stress testing is that the increased myocardial oxygen demand during exercise will produce clinical signs of ischemia (angina, ECG changes) in the setting of a flow-limiting stenosis that impairs myocardial blood supply. The diagnostic performance of this test varies greatly depending on the pretest likelihood of the population being studied. In patients with an intermediate pretest probability of CAD on the basis of symptoms and risk factors, exercise treadmill testing is useful as an initial screening test. However, the overall sensitivity of treadmill exercise testing is approximately 70%. A meta-analysis of more than 24,000 patients undergoing exercise stress testing and coronary angiography observed a mean sensitivity of 68% and a mean specificity of 77%. Accuracy was greatest in patients with multivessel coronary disease and those with left main or three-vessel coronary artery disease.6
Exercise Stress Myocardial Perfusion Imaging
Because exercise treadmill testing relies on changes in the 12-lead ECG as a surrogate of ischemia, the accuracy of the test is highly dependent on the baseline electrocardiogram. Abnormalities such as previous myocardial infarction (Q waves or left bundle branch block) or ST-T wave changes resulting from left ventricular hypertrophy or therapy with digoxin, hamper the ability to interpret ischemic changes accurately during exercise. In patients with an abnormal ECG, myocardial perfusion imaging adds significant diagnostic accuracy to the treadmill test for detecting CAD. Large studies have consistently shown that SPECT myocardial perfusion imaging with technetium-labeled agents, such as sestamibi, yields a greater than 90% sensitivity for the detection of coronary artery disease.7–9 False-negative scans tend to occur in the setting of single-vessel disease (particularly in the left circumflex artery distribution), a mild degree of stenosis (<70% occluded), or when the patient is unable to achieve the target heart rate or is taking antianginal therapy. These studies have also observed that the specificity of stress SPECT imaging is in the range of 68%. The less optimal specificity can be attributed to both referral bias (performing angiography in only those patients with abnormal scans), as well as false-positive scans with defects resulting from artifacts produced by soft tissue attenuation (diaphragm, breast) or patient motion.
Contemporary imaging techniques that employ ECG gating have facilitated the simultaneous assessment of myocardial perfusion and function. Examination of regional wall motion and thickening enhances the evaluation of a suspect area of hypoperfusion by providing the ability to distinguish true myocardial scarring from attenuation. Specificity can also be enhanced with the use of attenuation correction. These algorithms incorporate additional data obtained from an external radioactive source or computed tomography to create a soft tissue attenuation image of the chest, and then adjust the regional counts on the emission scan to correct for the loss of counts resulting from attenuation from overlying breast, diaphragm, and chest wall structures.10
Pharmacologic Stress Myocardial Perfusion Imaging
Dipyridamole is typically administered intravenously as a bolus injection over a period of 4 minutes, with peak effect occurring at 8 minutes, at which time the radiotracer is injected.11 A 4- to 6-minute continuous infusion of intravenous adenosine is now used more commonly.12,13 Both agents are equally effective in increasing myocardial blood flow threefold to fivefold over rest, which is similar to that produced by exercise. Although adenosine tends to achieve maximal flow in more patients than dipyridamole, side effects are more common with adenosine. Regadenoson is a new adenosine A2A receptor agonist that selectively increases coronary blood flow without producing side effects, such as hypotension, atrioventricular block, chest pain, and flushing, which are common with adenosine and dipyridamole.14–16 Regadenoson can be used as an alternative stressor in patients with lung disease, because its receptor selectivity may avoid the bronchospasm that can occur with other vasodilators.17,18
An intravenous infusion of dobutamine is also effective for pharmacologic stress. It is typically used as an alternative to vasodilator stress in patients with bronchospasm and pulmonary disease. The dobutamine infusion produces increased myocardial oxygen demand by increasing heart rate, blood pressure, and myocardial contractility.19
All of the currently used pharmacologic stress agents have similar abilities to produce flow heterogeneity and corresponding perfusion defects in the presence of a significant (>50% to 70%) stenosis of a coronary artery. A meta-analysis of dipyridamole SPECT imaging demonstrated a sensitivity of 89% and specificity of 65% for the detection of coronary artery disease. Dipyridamole and adenosine tests had essentially similar sensitivities and specificities. The sensitivity of dobutamine SPECT imaging in detecting CAD is approximately 80%, which is slightly lower than that of the vasodilator agents.20
Prognosis and Risk Stratification
Stable Angina
The goals of stress testing extend beyond the detection of coronary artery disease alone. For stress testing information to be useful, results must reliably identify patients of sufficiently low risk that further evaluation or intervention can be safely avoided, while also providing additional information to the clinical risk assessment when results are abnormal. The ability of both exercise and pharmacologic MPI to add incremental prognostic information to the clinical risk assessment in patients with known CAD has been evaluated in thousands of patients.21 Several large studies of patients with known or suspected CAD, including both men and women, have demonstrated the incremental prognostic value of a normal MPI.22–26
Perhaps of greatest clinical usefulness is that these studies have consistently shown that patients with a normal MPI have a subsequent annual rate of cardiac death and nonfatal myocardial infarction (MI) of less than 1%, even patients with known CAD.27,28 When the results are abnormal, the SPECT scan provides incremental prognostic information beyond that obtained by clinical, ECG, and stress testing combined. Moreover, the value of SPECT MPI in determining prognosis is such that the information obtained from subsequent coronary angiography does not provide any incremental prognostic information beyond that obtained by the SPECT results.29,30 The increase in the risk of hard events increases in proportion to the severity and extent of the perfusion defect when assessed by semiquantitative methods or automated quantitative approaches. The degree of ischemia on SPECT is associated with an increase in the short-term incidence of nonfatal MI, whereas scar size is more predictive of cardiac death.28 Important stress MPI variables that are predictive of future cardiac events include defects in more than one vascular territory (multivessel disease), transient left ventricular cavity dilation during stress,31 and increased pulmonary uptake of radiotracer32,33 (suggesting increased LV filling pressures), as well as other parameters outlined in Box 54-1. The prognostic ability of stress MPI is also enhanced by inclusion of ECG-gated SPECT, which permits assessment of LV global systolic performance, the most potent predictor of event-free survival, as well as analysis of regional wall motion and LV volumes.34–36
Medical Therapy versus Revascularization
One of the most important contributions of stress MPI in the clinical evaluation of patients with known or suspected CAD is the ability to provide prognostic information that can be used to guide further testing and therapies. Because the extent and severity of ischemia on a stress MPI study is directly proportional to the risk of future cardiac events, this information can be incorporated into the decision-making process concerning medical or revascularization therapies for a given patient. Patients with mild ischemic defects that are not high risk can generally be safely treated medically, whereas higher-risk findings (transient ischemic dilation, multivessel disease pattern, large territory at risk) should be strongly considered for more invasive evaluation and a lower threshold for revascularization. A 3-year follow-up study of patients with mild to moderate defects on stress SPECT myocardial perfusion imaging treated with medical therapy noted a 2% incidence of cardiac death or nonfatal infarction and less than a 5% rate of revascularization, suggesting that SPECT imaging is highly useful in identifying patients with CAD who have a sufficiently low risk for events that revascularization is not necessary.37
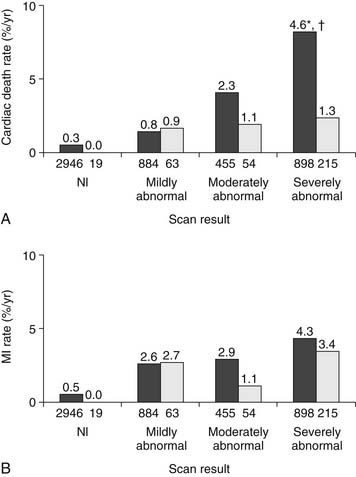
In contrast, the benefit of revascularization over medical therapy has been characterized in patients with high-risk stress SPECT MPI findings. Hachamovitch and colleagues28 followed 5183 patients after stress MPI for a mean of 2 years, and found that the rates of MI and cardiac death did not differ substantially between patients treated with medical therapy or revascularization after a normal or mildly abnormal scan. However, patients with severely abnormal scans had a significantly higher annualized cardiac death rate when treated medically (4.6% deaths per year) compared with those referred for revascularization (1.3% per year) (Fig. 54-3). Patients with a greater ischemic burden on imaging were also found to benefit more from revascularization than medical therapy, with serial imaging studies showing a greater reduction in reversible defect size in those treated with revascularization. A subsequent observational study of 10,627 consecutive patients with no prior infarct or revascularization demonstrated a survival advantage in patients with no or mild ischemia treated with medical therapy, with revascularization having prognostic benefit beyond medical therapy in patients with moderate to severe ischemia.38 More recently, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial39 showed no difference in the incidence of death or myocardial infarction in 2287 patients with stable angina randomized to optimal medical therapy or percutaneous revascularization. A substudy of this trial showed that the addition of percutaneous revascularization to optimal medical therapy resulted in more effective reduction of ischemia than medical therapy alone, with less angina.40 Moreover, regardless of treatment assignment, the degree of residual ischemia on follow-up perfusion imaging was proportional to the risk of subsequent cardiac events. Thus, it appears that revascularization can be employed selectively in patients with CAD based on the extent and severity of the ischemic burden. Patients with low-risk findings can be treated medically, whereas more extensive ischemia is best treated with more aggressive interventions.
These principles have been shown to have a favorable impact in terms of both patient outcomes and cost when applied clinically. Several large studies have focused on the clinical use of stress MPI to guide patient management and have shown that referral for coronary angiography is accordingly low (about 3.5%) after a normal stress MPI, but referrals increase to 60% when the stress MPI is moderately to severely abnormal.28,41,42 This demonstrates that clinicians can incorporate MPI into their practice appropriately, particularly when deferring further invasive evaluation when the MPI is normal. The use of stress MPI to guide patient management has also been demonstrated to be cost effective.
An “ischemia-guided” approach to managing patients with CAD has been shown to optimize the use of angiography and subsequent revascularization procedures.43 The Economics of Noninvasive Diagnosis (END) study44,45 compared differences in cost for 11,372 consecutive patients referred for either stress MPI or cardiac catheterization as the initial approach. Not only were the rates of subsequent myocardial infarction the same in the two groups, costs were significantly higher for the direct cardiac catheterization group as compared with the MPI ischemia-guided approach. Moreover, patients sent directly to cardiac catheterization were more likely to be treated with coronary revascularization in all subsets of risk when compared with those undergoing stress MPI initially. The ischemia-guided approach to stable angina was found to be less costly, with less interventions and similar cardiac event rates. These cost differences can be directly attributed to a decrease in apparently unnecessary procedures in patients with negative MPI results.
Preoperative Evaluation for Noncardiac Surgery
Perioperative cardiac events are an important cause of morbidity and mortality during and after noncardiac surgery, and they occur in more than 2% of patients undergoing these procedures. Much of this risk can be assessed preoperatively via clinical evaluation (a history of coronary artery or cerebrovascular disease, congestive heart failure, renal insufficiency, insulin-requiring diabetes, or high-risk surgical procedure) to identify patients at high risk for cardiac complications who might benefit from risk-reduction strategies (specific perioperative medical therapy or revascularization) and patients having sufficiently low risk that further evaluation before surgery is not necessary.46 However, patients with an intermediate risk for cardiac complications may benefit from further noninvasive testing to identify ischemia and provide further risk stratification prior to surgery.47 Provocative testing for ischemia is typically performed in such patients. However, exercise testing is not always feasible for patients with pulmonary disease or low exercise capacity, and in particular for those undergoing orthopedic or vascular surgery. Pharmacologic stress MPI has been shown to add significant information to preoperative risk stratification.48 It is recommended for those patients with intermediate clinical risk predictors (prior MI, diabetes, renal insufficiency), for those who have poor functional capacity, and for those undergoing a high-risk surgical procedure (emergent surgery, aortic or peripheral vascular surgery, prolonged procedures with anticipated large blood loss or volume shifts). When results of stress MPI are normal, the incidence of perioperative cardiac events has been shown to be approximately 1% for all procedures, including major vascular surgery.49 Although the severity and extent of ischemia remains an important predictor of adverse cardiac events during and after surgery, recent studies have shown that revascularization before noncardiac surgery does not appear to affect later outcome in terms of death and nonfatal myocardial infarction.50
ACUTE CORONARY SYNDROME
Territory at Risk and Infarct Size
Resting MPI has been used to determine the territory at risk during an acute coronary syndrome, and it can be used to assess the extent of myocardial salvage achieved with reperfusion strategies.51 Radiopharmaceuticals labeled with 99mTc can be injected at rest in the setting of an acute ST-segment elevation myocardial infarction on presentation. After initial treatment and stabilization with either pharmacologic or mechanical reperfusion, perfusion images can be acquired that represent the initial myocardial area at risk during the coronary occlusion. These images are then compared with those acquired at rest after a subsequent injection after reperfusion. The difference in the size of the defect between these two scans is the salvage index, representing the degree of myocardium that was rescued by reperfusion (i.e., the initial territory at risk minus the ultimate scar).52,53 Although this methodology is not used widely in clinical practice, it is a useful tool in the evaluation of therapies used in the treatment of acute MI. The salvage index has been validated as a surrogate marker of patient outcome, and it provides a quantitative means of comparing the efficacy of therapeutic strategies.54 The use of the infarct size, territory at risk, and the salvage index has played a central role in trials focusing on the efficacy of angioplasty52,55 and thrombolysis.56 This methodology can also assist in determining important clinical factors associated with reperfusion outcome, such as time to reperfusion.55
Myocardial infarct size can be measured quantitatively after MI and has been shown to be highly predictive of poor outcome.53,54,57,58 The ischemic burden on cardiac stress SPECT imaging is associated with future nonfatal myocardial infarction, whereas fixed perfusion defects and a depressed ejection fraction are predictive of future cardiac death.59 Quantification of the infarct size as a percentage of the left ventricle is an effective way to risk-stratify a patient’s risk for cardiac death in the near term. Studies using 99mTc SPECT after acute MI have observed that mortality within the subsequent 24 months was approximately 8% if the infarct encompassed more than 12% to 14% of the left ventricle.58,60
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree