Novel Systemic Therapy for Advanced Non-Small-Cell Lung Cancer
Anne S. Tsao
Merrill S. Kies
Lung cancer is the leading cause of cancer-related deaths worldwide. In the United States in the year 2007, an estimated 174,000 new cases of lung cancer will be diagnosed, with an additional 162,000 deaths.82 Non-small-cell lung cancer (NSCLC) comprises 85% of all the lung cancers and has a 15% overall survival rate at 5 years for all stages. In patients with metastatic disease, the 5-year overall survival remains less than 1%. Prior to the era of targeted therapy, chemotherapy was the mainstay of palliative treatment. In the front-line setting, patients were originally treated with single-agent therapy, but this evolved into using doublet regimens. Platinum-based doublets with third-generation chemotherapies came into common use and yielded median survival times of 9 months and 1-year overall survival rates of 30% to 40%.161
However, there remains some controversy over which is the optimal front-line doublet regimen, as Eastern Cooperative Oncology Group (ECOG) 1594 showed no survival differences between four different platinum-based regimens, but the cisplatin versus carboplatin (CISCA) meta-analysis suggested some survival benefit to using cisplatin over carboplatin.3 In addition, although platinum-based doublets are commonly used as the standard of practice for chemo-naive patients, additional large randomized trials and meta-analyses have shown that non–platinum-based doublets with third-generation chemotherapies are as efficacious as platinum-based therapies.93,189 Prior to 2005, it was therefore the standard of practice in a patient with good performance status to use a doublet combination regimen in the front-line setting of advanced NSCLC treatment, with non–platinum-containing regimens being used as an alternative to platinum-based combinations.140
The era of targeted therapies has changed the landscape of lung cancer treatment. In 2005, the results of ECOG 4599 were presented and established a new standard practice in a certain population of lung cancer patients.158 The addition of bevacizumab, an antiangiogenic agent, improved the median survival over that for the use of chemotherapy alone in patients with metastatic NSCLC. Owing to significant toxicities, bevacizumab is recommended for use only in patients with non–squamous cell histology, no brain metastasis, and no hemoptysis. The nonplatinum or platinum-based doublet remains the standard of care for the front-line treatment of patients who cannot receive bevacizumab.
The addition of novel therapies has also affected second- and third-line therapy for patients with advanced metastatic NSCLC. Three agents currently approved for use by the U.S. Food and Drug Administration (FDA) are commonly used in the salvage setting: docetaxel, pemetrexed, and erlotinib. To date, there have not been any definitive studies suggesting that any one of these agents should be used over another. However, with advances in laboratory technology, it is becoming clear that individualized therapy based on clinical and tumor biomarker prognostic factors can optimize treatment.
At this time, clinical characteristics such as sex, ethnicity, smoking status, and development of a rash may predict a better clinical response or survival with particular novel agents. However, we currently know that specific genetic mutations are prognostic of either an improved response or even resistance to therapy. Future treatment algorithms of solid tumor oncology will likely become molecularly based, with targeted agents given in combination and sequenced depending on the molecular profile of the patient’s tumor. The remainder of this chapter focuses on the new novel biologic agents.
Targeting the Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR) was one of the first targets of novel therapeutics developed successfully in lung cancer. EGFR is a 170-kDa transmembrane glycoprotein that is encoded by the c-erbB-1 protooncogene.22,23 The receptor is expressed on the surface of normal epithelial-derived cells, such as skin, liver, and gastrointestinal tract.162,187 However, EGFR protein expression has been identified in human solid tumors, including NSCLC.41,156 Numerous studies report EGFR overexpression in 40% to 80% of patients with NSCLC.53,155,156 Studies have revealed that mutations in the adenosine triphosphate binding region of the receptor may confer a greater likelihood of response to tyrosine kinase inhibitors of EGFR, and gene
amplification may also predict an improved survival outcome when these agents are used.
amplification may also predict an improved survival outcome when these agents are used.
EGFR Structure and Function
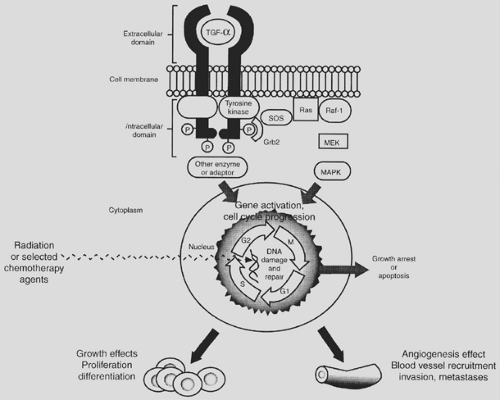
EGFR is composed of three domains: an extracellular ligand-binding domain, a transmembrane lipophilic domain, and an intracellular protein tyrosine kinase domain (Fig. 118-1).66 The ligands for EGFR include EGF, transforming growth factor-alpha (TGF-α), heparin-binding EGF, amphiregulin, and betacellulin.156 Once a ligand binds to EGFR, the ligand–receptor complex dimerizes, internalizes, and activates intracellular protein kinases via autophosphorylation.21,202,203 This subsequently leads to a signal transduction cascade that enables cell proliferation, differentiation, and survival. In lung cancer, EGFR overexpression, gene amplification, or gene mutation can lead to increased tumor cell proliferation, invasion, differentiation, invasion and metastasis, and inhibition of apoptosis.2,32,40,43,63,99,139,145,148,169,178,201 EGF and other growth factors (i.e., TGF-α) can also stimulate angiogenesis (the formation of new blood vessels), which facilitates tumor cell growth and metastasis.50
EGFR Molecular Predictors of Prognosis and Treatment Response
EGFR has been analyzed as protein expression by immunohistochemistry (IHC), gene amplification by fluorescence in situ hybridization (FISH), and gene sequencing; it has been studied in a retrospective fashion in tumor tissue to predict treatment response and disease progression. Although controversial, higher expression of EGFR protein appears to correlate with a worse response to chemotherapy and possibly with poorer survival.49,155 EGFR overexpression has not been correlated with response to treatment with tyrosine kinase inhibitors.54,95
Gene amplification or “FISH-positive” status has been correlated with improved survival when patients are treated with the new EGFR-targeted agents.10 In addition, critical mutations in the adenosine triphosphate (ATP) pocket of the tyrosine kinase binding region were recently reported in patients with NSCLC.109,131 These mutations are suspected to cause constitutive activation of the receptor and to confer susceptibility to EGFR tyrosine kinase inhibitors.109 Approximately 10% to 15% of NSCLC patients will have a gene mutation, and it is estimated that 85% of the mutation carriers will have significant clinical
responses to the EGFR tyrosine kinase inhibitors. EGFR exons 18 through 21 encode for structures around the ATP binding cleft within the tyrosine kinase domain.109,131,132,171 This is the binding area for the EGFR tyrosine kinase small-molecule inhibitors gefitinib and erlotinib. However, screening for EGFR mutations is not recommended, as some retrospective studies have not correlated a survival benefit with the presence of the EGFR mutation.36,190
responses to the EGFR tyrosine kinase inhibitors. EGFR exons 18 through 21 encode for structures around the ATP binding cleft within the tyrosine kinase domain.109,131,132,171 This is the binding area for the EGFR tyrosine kinase small-molecule inhibitors gefitinib and erlotinib. However, screening for EGFR mutations is not recommended, as some retrospective studies have not correlated a survival benefit with the presence of the EGFR mutation.36,190
As the field has developed further, secondary mutations in the EGFR gene have been determined to nullify the response to EGFR tyrosine kinase inhibitors. Also, mutations in the K-ras gene are negative predictors of response to these novel agents. K-ras mutations are mutually exclusive of EGFR mutations and are a reliable negative predictor of response.
Therapeutic Strategies to Target EGFR
There are various approaches to block the EGFR (Table 118-1). One approach is targeting the extracellular portion of the receptor by inhibiting the ligand or binding and blocking the ligand-binding domain of the EGFR.8,120,144 Normally, ligand (i.e., EGF, TGF-α) binding induces receptor dimerization, autophosphorylation, and then downstream signaling to the cell nucleus to initiate cell proliferation. Blockade of the extracellular region prevents the signal transduction cascade from occurring. Several intravenous antibodies, such as cetuximab, are under investigation in NSCLC.9,52 Small-molecular inhibitors or tyrosine kinase inhibitors (e.g., gefitinib, erlotinib) act intracellularly on the cytoplasmic domain of the EGFR and are oral agents that are taken daily. Agents in this class of novel drugs have come furthest along in clinical trial development and have been the only EGFR targeting agents approved for use in NSCLC by the FDA. Ligand/toxin conjugates utilize both the ligand and immunoconjugates bind extracellularly to the EGFR and deliver the toxin into the cell after internalization of the ligand/toxin complex.4,24,163 A new method under development uses antisense oligonucleotides to prevent the translation of mRNA into the protein.67,121,154
Clinical Trials—EGFR Tyrosine Kinase Inhibitors
Gefitinib (ZD-1839, Iressa), a synthetic anilinoquinazoline inhibitor, is an orally active, selective EGFR-tyrosine kinase inhibitor that blocks signal transduction pathways involved in the proliferation and survival of neoplastic cells. Preclinical studies have demonstrated specificity in inhibiting EGFR tyrosine kinase autophosphorylation and shown a dose-dependent effect on apoptosis, growth inhibition, reduced cell line proliferation, and inhibition of growth factors (TGF-α, bFGF, VEGF) in tumor cell lines (e.g., colon, breast, ovarian, gastric).114
Several phase I trials (n = 254) were therefore initiated in various tumors, including NSCLC (n = 99), in the United States, Europe, and Japan.6,46,60,94,193 These trials evaluated doses ranging from 150 to 800 mg/day and reported significant side effects at daily doses >600 mg. Therefore two doses were selected for further evaluation: 250 and 500 mg/day. These early trials in NSCLC were promising, with some responders, and one-third of patients had disease stability.94,193 Two phase II trials were therefore initiated and called the Iressa Dose Evaluation in Advanced Lung Cancer (IDEAL I and II) trials.
In IDEAL I, gefitinib was given as second- or third-line monotherapy for patients with advanced NSCLC.54 There were 43 participating centers from Europe, Australia, South Africa, and Japan. Patients were stratified into Japanese and non-Japanese patients. The patients (n = 210) were randomized to receive gefitinib at a daily oral dose of either 250 or 500 mg. For the 250- and 500-mg doses, the response rates were 18.4% and 19%, respectively. The number of prior therapies (e.g., second or third line) did not affect the response rate. The disease control rate was 54.4% for the 250-mg group compared with 51.4% for the 500-mg group. The median durations of response were 3.2 and 4.6 months for the two groups, respectively. Median progression-free survivals for the 250- and 500-mg groups were 2.7 and 2.8 months, respectively, and the percentages of patients who were progression-free at 4 months were 29% and 39%. The median overall survival times were 7.6 months for the 250-mg group and 8.0 months for the 500-mg group. In this trial, a tumor response was predictive of survival; patients with a tumor response had median survival times of 13.3 months and 10.6 months, respectively.
The IDEAL II trial was based in North America and was conducted for patients who had undergone two or more unsuccessful chemotherapy treatments, including a platinum-containing regimen and docetaxel. In this study, 12% of the patients responded at the 250-mg dose and 9% at the 500-mg dose. The higher dose appeared to be associated with more rash and diarrhea, but an improvement in symptoms was noted in approximately 40% of patients in both groups.95 On the basis of the prior studies (IDEAL) using EGFR tyrosine kinase inhibitors, approximately 9% to 12% of European patients and 18% to 19%
of Asian patients with NSCLC experienced a tumor response to gefitinib. Subpopulation analyses revealed that responders were more likely to be female, to be nonsmokers, and to have adenocarcinoma with bronchioloalveolar features. On the basis of these trials, gefitinib was approved by the FDA for use in patients after platinum failure. However, gefitinib is no longer utilized in the United States, as the phase III ISEL trial did not show an overall improvement in survival in patients with metastatic NSCLC, and ISEL was therefore considered a negative trial. The subgroup analysis from this trial did report a survival benefit for never-smokers and adenocarcinoma patients, but it was a negative study for the overall lung cancer population. At the same time the ISEL results were reported, the results of the BR.21 trial using erlotinib monotherapy became known, and this trial was a positive study for overall survival. Erlotinib is currently the only EGFR tyrosine kinase inhibitor in wide usage in the United States for NSCLC.
of Asian patients with NSCLC experienced a tumor response to gefitinib. Subpopulation analyses revealed that responders were more likely to be female, to be nonsmokers, and to have adenocarcinoma with bronchioloalveolar features. On the basis of these trials, gefitinib was approved by the FDA for use in patients after platinum failure. However, gefitinib is no longer utilized in the United States, as the phase III ISEL trial did not show an overall improvement in survival in patients with metastatic NSCLC, and ISEL was therefore considered a negative trial. The subgroup analysis from this trial did report a survival benefit for never-smokers and adenocarcinoma patients, but it was a negative study for the overall lung cancer population. At the same time the ISEL results were reported, the results of the BR.21 trial using erlotinib monotherapy became known, and this trial was a positive study for overall survival. Erlotinib is currently the only EGFR tyrosine kinase inhibitor in wide usage in the United States for NSCLC.
Table 118-1 Mechanism of Action and Examples of EGFR Inhibitiona | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Gefitinib was also evaluated in combination with chemotherapy. The first pilot trials included gefitinib combined with carboplatin/paclitaxel in chemo-naive patients with stage IIIB/IV NSCLC.117 In part 1 of the study (dose-escalating), intermittent gefitinib (250 or 500 mg) was combined with carboplatin and paclitaxel; in part 2, the safest dose of gefitinib determined in part 1 (500 mg) was given continuously with carboplatin and paclitaxel. In part 1, no dose-limiting toxicities were observed with the 250-mg dose; at 500 mg, however, one patient developed a grade 3 rash. No new, increased, or cumulative toxicities have been observed; furthermore, no significant pharmacokinetic drug interactions have been observed, although there was a slightly reduced clearance of gefitinib in patients receiving chemotherapy, likely secondary to effects on hepatic metabolism. The combination of gefitinib (250- and 500-mg doses) and carboplatin/paclitaxel or gemcitabine/cisplatin was subsequently evaluated for survival benefits in two large phase III placebo-controlled studies (called the INTACT trials) in chemo-naive patients with NSCLC.57,69 INTACT I enrolled 1,093 patients and INTACT II enrolled 1,037 patients. Both of these studies failed to reach their primary endpoint and showed no survival benefit of adding gefitinib to chemotherapy.
Erlotinib (OSI-774, Tarceva) is another oral quinazoline with potent activity against EGFR autophosphorylation and tumor cell proliferation.122 In a phase II study (n = 57), erlotinib at a 150-mg oral daily dose was given to patients with NSCLC who had EGFR-expressing tumors and had undergone unsuccessful platinum-based therapy.15 For 56 patients evaluable for tumor response, the response rate was 14.3%, with a median survival of 257 days. One patient had a complete response, 7 had partial responses, and 15 had stable disease. The main toxicity was consistent with those of prior gefitinib studies, with rash and diarrhea being the most common adverse events.136 Erlotinib monotherapy (150-mg daily dose) was then evaluated in the large phase III NCI-CTG BR.21 trial.168 This trial randomized 731 patients 2:1 to erlotinib monotherapy (n = 488) or placebo (n = 243), with the primary endpoint of overall survival. All patients had received one or two unsuccessful prior therapies, had an ECOG performance status of 0 to 3, and had locally advanced stage IIIB or metastatic stage IV disease. Patients who received erlotinib had higher response rates, disease stabilization, and better progression-free and overall survival rates. The response rate with erlotinib was 9%; disease stabilization was 35%, with a median duration of response of 34.3 weeks. Erlotinib prolonged progression-free survival to 9.9 weeks over placebo at 7.9 weeks (HR 0.59, p<0.001). The median overall survivals were 6.7 months and 4.7 months, respectively (HR 0.73, p<0.001). This translated into 1-year survival rates of 31.2% with erlotinib and 21.5% with placebo. The subgroup analysis from BR.21 showed that all patients appeared to derive a survival benefit from erlotinib. The FDA therefore approved erlotinib for use in the United States for second- and third-line therapy in patients with metastatic NSCLC.
Erlotinib was also combined with chemotherapy in two large phase III randomized trials called TRIBUTE and TALENT.55,73 TRIBUTE combined erlotinib with carboplatin/paclitaxel, while TALENT combined erlotinib with cisplatin/gemcitabine. In both studies, there was no survival benefit with the addition of erlotinib to front-line chemotherapy for the general population. Erlotinib was therefore not approved for use in combination with chemotherapy. However, subgroup analysis of both studies revealed that never-smokers appeared to have a survival benefit from the use of erlotinib. Owing to the small numbers of patients, this result did not reach statistical significance.
EGFR Tyrosine Kinase Inhibitor Toxicity Profile
The most common toxicities of the EGFR inhibitors include rash and diarrhea. Most patients with the rash experience grade 1 to 2 toxicity in terms of the National Cancer Institute Common Toxicity Criteria (NCI-CTC); only a small percentage of patients experience grade 3 to 4 skin rash. Approximately half the patients will experience grade 1 to 2 diarrhea, but only 6% will have grade 3 to 4 diarrhea. In addition, there is a small risk of interstitial lung disease, hepatotoxicity, and interaction with other medications that utilize the P-450 system. Rarely, ophthalmic toxicities can occur.
The etiology of the skin rash remains unclear. Several theories postulate that it arises from inhibition of EGFR expressed on normal keratinocytes in the basal layer of the epidermis,137 owing to an inflammatory reaction to dysregulated keratinocyte proliferation or differentiation or to superinfection of the inflammatory reaction in affected areas.138 Roughly 10% of patients will discontinue therapy because of the rash.138 Skin rash can manifest as acne, pruritus, or dry skin.
To limit the side effects of rash and diarrhea, the first step is to ensure that patients take the erlotinib on an empty stomach, at least 1 hour prior to consuming food or at least 2 hours after eating. When erlotinib is taken with food, the bioavailability is altered, with a resulting increase in side effects and without the benefit of additive antitumor activity. There are no evidence-based recommendations on the treatment of rash or diarrhea, but early intervention and education are general requirements. The rash can be treated with topical antibiotic creams, oral antibiotics, topical steroid creams, or calamine lotion, or (only in the most severe cases) with oral steroids. The diarrhea is often effectively treated with over-the-counter antidiarrheal medication.
Interstitial lung disease (ILD) occurred in 0.8% of patients receiving erlotinib, but in the Japanese population, gefitinib induced a 2.7% rate of ILD. ILD presents with hypoxia, acute shortness of breath, cough, and fever. Hepatotoxicity is rare, but asymptomatic increases in the transaminases can occur. In most cases, this will resolve spontaneously; however, dose reduction or interruption is recommended if abnormal results of the liver function tests are persistent or severe. Because erlotinib is
primarily metabolized through the liver, it can interact with other medications. CYP4A inhibitors decrease erlotinib metabolism and increase its plasma concentration. In rare cases, INR elevations can occur when erlotinib and warfarin are taken together. Erlotinib should not be given to pregnant or nursing women; it is classified as pregnancy category D. Among the ophthalmic adverse events that can occur, albeit rarely, conjunctivitis, blepharitis, keratitis, eye pain, dry eyes, and corneal erosion are possible.
primarily metabolized through the liver, it can interact with other medications. CYP4A inhibitors decrease erlotinib metabolism and increase its plasma concentration. In rare cases, INR elevations can occur when erlotinib and warfarin are taken together. Erlotinib should not be given to pregnant or nursing women; it is classified as pregnancy category D. Among the ophthalmic adverse events that can occur, albeit rarely, conjunctivitis, blepharitis, keratitis, eye pain, dry eyes, and corneal erosion are possible.
Monoclonal Antibodies to EGFR
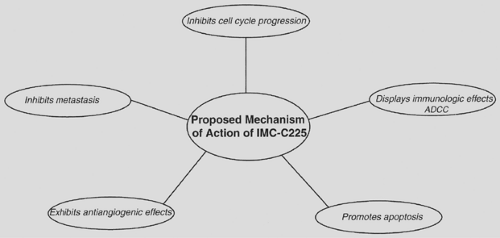
Cetuximab (IMC-C225) is a human:murine chimeric anti-EGFR IgG1 monoclonal antibody that competes with EGF for binding to the EGFR and inhibits EGF-induced tyrosine kinase–dependent phosphorylation.42 Cetuximab binds to EGFR, promotes internalization of the receptor, and facilitates its degradation, thereby translating into antitumor efficacy. In addition to its inhibitory effect on cell cycle progression, promotion of apoptosis, and antiangiogenesis, cetuximab can activate immune effector cells via antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity (Fig. 118-2).72 Cetuximab has also been shown to reduce metastasis formation by downregulating the expression of matrix metalloproteinases.130
Cetuximab has been approved for use by the FDA for colorectal and head and neck squamous cell cancers. The initial phase I trials in various solid tumor types demonstrated adverse events of hypersensitivity and skin rash.7,172 When combined with cisplatin chemotherapy, cetuximab contributes to side effects of fatigue, peripheral neuropathy, and orthostatic hypotension. In NSCLC cell lines, cetuximab inhibits growth in high EGFR-expressing NSCLC cells. When combined with radiation therapy or chemotherapy, the regimens inhibit growth in high/moderate EGFR-expressing cells. Several clinical trials have evaluated cetuximab in NSCLC as monotherapy and in combination with chemotherapy. Cetuximab is administered intravenously weekly, with a loading dose of 400 mg/m2 given during week 1 and 250 mg/m2 administered during all subsequent weeks.
As monotherapy, cetuximab has been given in a phase II trial to 66 previously treated NSCLC patients.65 This study reported a 4.5% response rate and stable disease in 30% of the patients. The median time to progression was 2.3 months and median overall survival was 8.9 months; the 1-year survival rate was 43.9%. The most common adverse event was the skin rash, and in the tumor specimens that were available, there was no correlation of EGFR status with tumor response to therapy.
Cetuximab has been combined with chemotherapy, as prior studies in colorectal and head and neck cancer have demonstrated reversal of chemotherapy resistance with the addition of cetuximab.19,29,157 In the front-line metastatic setting, five clinical trials have combined cetuximab with carboplatin/paclitaxel,88,185 carboplatin/gemcitabine,147 and cisplatin/vino- relbine.149,195 The cetuximab/carboplatin/paclitaxel regimen was first studied in 31 patients and yielded a 26% response rate, median time to progression of 5 months, and median overall survival of 11 months.185 The Southwest Oncology Group (SWOG) conducted a randomized trial (SWOG 0342) comparing cetuximab administered concurrently or sequentially after carboplatin/paclitaxel.88 Following completion of chemotherapy, all patients received cetuximab weekly as maintenance for a year. SWOG 0342 enrolled 225 patients and reported that both arms had median progression-free survival of 4 months. The concurrent arm had a slightly higher median survival (10 months versus 9 months) and 1-year survival rate (49% versus 43%). There was no significant difference in toxicity between the two arms except for skin rash corresponding to cetuximab administration. SWOG is planning a new phase II trial utilizing carboplatin, paclitaxel, cetuximab, and bevacizumab
followed by cetuximab and bevacizumab maintenance (SWOG 0536).
followed by cetuximab and bevacizumab maintenance (SWOG 0536).
The cetuximab/carboplatin/gemcitabine regimen was used in 35 patients with a 28% response rate, median progression-free survival of 5.3 months, and median overall survival of 10.3 months.147 The two trials using cetuximab/cisplatin/vinorelbine were randomized and compared the triplet regimen to an arm containing chemotherapy alone. In the first trial of 86 patients, the addition of cetuximab improved the response rate (35% versus 28%), median progression-free survival (4.8 months versus 4.2 months), and median overall survival (8.3 months versus 7 months). The FLEX trial was of identical design but is a multicenter phase III trial and has completed its accrual with 1,124 patients. Results from the FLEX trial are pending at this time.
In the salvage setting, cetuximab has been combined with docetaxel chemotherapy in 54 patients who underwent unsuccessful platinum-based therapy during treatment or within 3 months of completing treatment.90 Forty-seven patients were evaluable, with a 28% response rate, median time to progression of 3 months, and median overall survival of 7.5 months. Cetuximab is also being investigated in the setting of unresectable stage III locally advanced NSCLC in combination with radiation in two large cooperative group trials, RTOG 0342 and CALGB 30407. The Radiation Therapy Oncology Group (RTOG) in trial 0342 combined cetuximab with six cycles of weekly paclitaxel (45 mg/m2) and carboplatin (AUC 2) and concurrent radiation therapy (63 Gy in 35 fractions). After completion of the chemoradiation treatment, additional cycles of full-dose systemic therapy are given. The Cancer and Leukemia Group B (CALGB) 30407 trial is a phase II trial combining carboplatin and pemetrexed with concurrent radiation (70 Gy over 7 weeks) with or without cetuximab. Four cycles of consolidation therapy with pemetrexed are sequenced after completion of the chemoradiation.
The main toxicity encountered in all the chemotherapy and cetuximab trials was skin rash. Additional toxic effects included fatigue, acne-like rash, myalgia, and neuropathy. Patients with a rash were likely to have tumor response to cetuximab and chemotherapy. However, there were no other clinical or molecular prognostic factors, such as EGFR overexpression or mutation, that consistently correlated with treatment response.65
The role of cetuximab in NSCLC remains unclear. Results of monotherapy and regimens using chemotherapy in combination with cetuximab in the metastatic setting have been largely unimpressive to date and have not established a place for cetuximab in this setting. The SWOG 0342 and FLEX trials are still pending, but it is unlikely that results for these will be significantly different. The patient population with unresectable stage III disease may benefit from the addition of cetuximab, as it is known that cetuximab sensitizes tumor cells to radiation and may prevent tumor cell repopulation. It is hoped that RTOG 0342 and CALGB 30407 will provide clarity on the usage of cetuximab in NSCLC.
The EGFR inhibitors were the first targeted therapies to affect the treatment of NSCLC. Currently, second-generation EGFR targeted agents are under investigation and may lead to continued advances. Some of the promising agents include EKB-569, HKI-272, CI-1033, and zactima (ZD6474). EKB-569 is an irreversible inhibitor of EGFR tyrosine kinase activity and has been evaluated in phase I and II trials. HKI-272 is an irreversible inhibitor of EGFR and HER-2 and is currently under investigation in a phase II international study. CI-1033, an irreversible pan-ErbB inhibitor, has been studied in phase I and II trials as monotherapy and also in combination with chemotherapy (docetaxel, carboplatin/paclitaxel). The results of these studies are still pending. Zactima or ZD6474 is a dual kinase inhibitor that targets EGFR and vascular endothelial growth factor receptor. Further information will be discussed in the anti-angiogenic therapies section. Although the first-generation EGFR inhibitors had a significant effect on patient therapy, further progress is needed.
Targeting Angiogenesis
Angiogenesis, or the formation of new blood vessels from preexisting vessels, is necessary for the growth and development of solid tumors (>1–2 mm3) and metastases.48,77,91 Angiogenesis is a multistep process: endothelial cells, which line the lumen of the blood vessels, first degrade the basement membrane, migrate through to form a sprout, and then proliferate to extend the new vessel. Tumor metastasis involves five main steps: (a) angiogenesis, (b) adhesion to endothelial cell basement membrane, (c) proteolytic destruction of the basement membrane, (d) migration to secondary sites, and (e) proliferation at the secondary sites.104 Figure 118-3 depicts the process of tumor angiogenesis, which is associated with an increased incidence of metastases, worsening prognosis, and reduced survival in NSCLC.27 The increased frequency of metastases with adenocarcinoma may be attributed to the higher microvessel density than in other forms of NSCLC (i.e., squamous cell carcinoma).174
Angiogenesis is stimulated by various angiogenic growth factors, including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). However, a balance between activators and inhibitors of angiogenesis maintains tumor vascular homeostasis. Tumors can also stimulate endogenous inhibitors of angiogenesis.25 Some of these inhibitors are angiostatin,129 endostatin,128 and thrombospondin.59 The angiogenic process is determined by the net balance of angiogenic inducer activity over angiogenic inhibitor activity. Additional inhibitors of the angiogenic switch include platelet-factor 4, 16-kDa prolactin, and interferon αβ. Cancer-associated vasculature differs from normal blood vessels; it is typically tortuous, leaky, and disorganized. This disordered blood flow leads to areas in the tumor that are hypoxic and acidic, which in turn selects for more malignant clones.81 Antiangiogenesis therapy has several goals. The first is to normalize aberrant tumor vasculature by remodeling leaky vessels. The second goal is to decrease interstitial fluid pressure to thereby increase chemotherapy uptake into the tumor; this can also increase tumor oxygenation and sensitization to radiation therapy. Last, antiangiogenesis therapy seeks to prevent any further neovascularization.81
VEGF is an important endothelial cell–specific mitogen involved in tumor neovascularization.45 VEGF has two identified receptors: Flt-1 (fms-like tyrosine kinase-1) and Flk-1/KDR (fetal liver kinase-1/kinase domain region). Both receptors for VEGF are upregulated on tumor vasculature as well as in other actively developing tissues and in wound healing. VEGF expression is associated with high microvessel counts and therefore increasing vascularity.179 Furthermore, expression of VEGF and its Flk-1/KDR receptor has been found in the intestinal form of gastric cancer and correlated with metastases to the liver.181
Upregulation of VEGF and its receptors can also be identified in the blood vessels of human colorectal tumors implanted into mice.196 The VEGF receptor Flk-1/KDR has been identified in other mammary, ovarian, lung, and glioma tissues, indicating roles for VEGF in these forms of cancer and in the identification of this receptor as a potential target.116 High VEGF expression may be associated with a worse prognosis in lung cancer.76
Upregulation of VEGF and its receptors can also be identified in the blood vessels of human colorectal tumors implanted into mice.196 The VEGF receptor Flk-1/KDR has been identified in other mammary, ovarian, lung, and glioma tissues, indicating roles for VEGF in these forms of cancer and in the identification of this receptor as a potential target.116 High VEGF expression may be associated with a worse prognosis in lung cancer.76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree