Novel Strategies for Lung Cancer Immunotherapy
Dominik Rüttinger
Rudolf Hatz
Karl-Walter Jauch
Bernard A. Fox
For a long time, lung cancer was not considered an immune-sensitive malignancy. For this reason and owing to insufficient knowledge of relevant tumor antigens, lung cancer immunotherapy lags behind similar efforts in melanoma as well as renal cell and prostate cancer. However, there is increasing evidence that non-small-cell lung cancer (NSCLC) and small cell lung cancer (SCLC) can evoke specific humoral and cellular antitumor immune responses. With increasing knowledge about the link between the induced immune response and a resulting objective clinical response, targeted agents may hold great promise in sequence and/or combination with other antitumor treatment modalities such as surgery, chemotherapy, and radiation. Lung cancer is still the deadliest cancer in the world; it killed an estimated 163,390 people in the United States in 2007, including 15,000 to 20,000 “never smokers.”81 These numbers clearly demonstrate that despite progress in the treatment of this disease over the past two decades, there are still few long-term survivors: only about 10% of all lung cancer patients will ever be cured of this devastating disease.42
The optimal chemotherapy regimens available (platinum-based regimens consisting of paclitaxel, docetaxel, gemcitabine, or vinorelbine) have demonstrated only limited activity, with median survival times of less than 11 months and 1-year survival rates of 31% to 36%. Most large randomized trials assessing systemic chemotherapy for lung cancer have also included patients with metastatic disease, who represent the majority of NSCLC patients.17,75,76 But in the adjuvant setting as well, systemic chemotherapy has turned out to be only modestly effective, with considerable toxicity. In summary, systemic chemotherapy for lung cancer of all stages is given with limited effect on survival and palliative intent in metastatic disease. Based on these facts, currently a wide variety of immunotherapeutic agents are being tested in lung cancer. Although any categorization of immunotherapeutic approaches is difficult because of frequent overlaps in mechanisms of action, four main approaches that may be classified as “immunotherapy” can be distinguished (Figure 121-1):
Antibody-based immunotherapy
Tyrosine kinase inhibitors (as part of anti-EGFR and anti-VEGFR strategies)
Therapeutic lung cancer vaccines
Adoptive cell transfer
This chapter reviews strategies based on the humoral and cellular immune system that are already in clinical use or well progressed in early clinical trials. Because NSCLC accounts for approximately 80% of all lung cancers, the focus is mainly on immunotherapy in NSCLC.
Antibody-Based Immunotherapy
For a long time, only a single monoclonal antibody (mAB), muromonab-CD3 (Orthoclone, OKT3), was licensed by the U.S. Food and Drug Administration (FDA). The general reasons for the low therapeutic efficacy of mABs in the fight against cancer were their immunogenicity in humans due to their murine origin, their short in vivo survival, and their failure to kill target cells efficiently because they failed to fix human complement or elicit antibody-dependent cellular cytotoxicity (ADCC) with human mononuclear cells. In addition, many mABs were not directed against cell-surface structures such as growth factor receptors. With the development of human or humanized antibodies and the discovery of antigenic cell-surface targets, these problems have been mostly overcome. Today, at least 9 mABs have received FDA approval for cancer therapy38 and over 400 others are being tested in clinical trials for various indications.24,38 In lung cancer, a primary focus was on mABs targeting the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor (VEGF).14,32 The most advanced in development are:
anti-EGFR mAB cetuximab (Erbitux)
anti-VEGF mAB bevacizumab (Avastin)
Antibodies Targeting Growth Factors
In fighting cancer, the blocking of growth factors and their receptors seems an obvious strategy because they are known to augment tumor cell proliferation and invasion and their overexpression in many solid malignancies has been associated with a more aggressive course and poor survival.64 C-erb B-1 and c-erb B-2 are the two growth factor receptor families that have been studied most extensively. C-erb B-1 is better known under the name HER1, or epidermal growth factor receptor (EGFR). HER2 is the more common name for c-erb B-2.
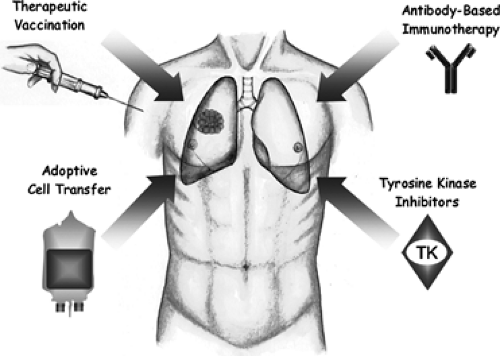 Figure 121-1. Immunotherapeutic approaches in lung cancer. Currently applied treatment strategies based on the humeral and cellular immune system consist mainly of active-specific immunotherapy, i.e., therapeutic vaccination and antibody-based immunotherapy with a primary focus on antibodies targeting the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor (VEGF). To a lesser extent, adoptive cell transfer (e.g., lymphokine-activated killer [LAK] cells) is in the early phase of clinical evaluation. Strictly speaking, tyrosine kinase (TK) inhibitors are not components of the humeral or cellular immune system; however, they are mentioned here because some have progressed in clinical development and are part of anti-EGFR and anti-VEGFR strategies (covered in Chapter 118). TK are small molecules competing with ATP for binding to the intracellular catalytic domain of tyrosine kinase. |
Anti-EGFR (anti-C-erb B-1) Monoclonal Antibodies
Cetuximab (Erbitux), a chimeric human:murine form of the original mAB 225, has demonstrated safety and was well tolerated in early-phase clinical trials, but low patient numbers currently do not allow for final assessment of its therapeutic efficacy in lung cancer. A 28% partial response rate and 17% of patients with stable disease were observed in a phase II trial with the combination of cetuximab and docetaxel, which exceeds response rates usually seen with docetaxel alone.39 In another phase II trial, patients with recurrent or progressive NSCLC were treated with cetuximab after receiving at least one prior chemotherapy regimen.27 The response rate for all patients (n = 66) was 4.5%, and the stable disease rate was 30.3%. The median time to progression for all patients was 2.3 months and median survival time was 8.9 months. The authors of this study concluded that, although the response rate with single-agent cetuximab in this heavily pretreated patient population with advanced NSCLC was only 4.5%, the disease control rates and overall survival seemed comparable with those of pemetrexed, docetaxel, and erlotinib in similar groups of patients. More phase I/II clinical trials have been conducted on the use of cetuximab in combination with systemic chemotherapy and/or radiation therapy, confirming low toxicity but also limited clinical benefit.6,36,65 Grade 3 toxicities associated with the use of cetuximab were fatigue, infection, and papulopustular rash. Development of the rash, usually located on the face and upper torso, has been related to a clinical response and may serve as a surrogate marker for cetuximab activity.58
Other anti-EGFR mABs currently in development include panitumumab (ABX-EGF), matuzumab (EMD 72000), pertuzumab (2C4), and MDX214.32,79 A phase I trial of matuzumab in combination with paclitaxel has been reported in 18 patients with EGFR-positive advanced NSCLC.41 In this study, objective responses were seen in 4 of 18 (23%) patients. A randomized phase II trial is currently ongoing in second-line NSCLC using matuzumab in combination with pemetrexed. A randomized phase II trial of carboplatin/paclitaxel with or without panitumumab in 166 patients with previously untreated advanced stage IIIB/IV NSCLC did not find any benefit for the panitumumab arm with regard to response rates, time to disease progression, or median survival time.10
More early-phase clinical trials with these agents in patients with lung cancer are currently ongoing with results pending.
Anti-HER2 (anti-c-erb B-2) Monoclonal Antibodies
Trastuzumab (Herceptin), a humanized monoclonal antibody that targets the HER2 receptor, has been approved for metastatic breast cancer. However, to date, it has failed to demonstrate clinical efficacy in patients suffering from lung cancer.8,19 Only few authors suggest further investigation of trastuzumab in HER2-positive lung cancer.44 In contrast, pertuzumab (rhu mAB 2C4), a mAB designed to inhibit the dimerization of HER2 with EGFR and other HER tyrosine kinases and therefore independent of HER2 overexpression, is currently under evaluation in NSCLC in early-phase clinical trials.32 In 43 patients with previously treated NSCLC, monotherapy with pertuzumab failed to induce objective clinical responses, but tumors of 27% of the patients showed significantly lower glucose metabolism as assessed by positron emission tomography.30 According to the authors, further clinical development of pertuzumab will focus on combination with other drugs active in NSCLC.
Monoclonal Antibodies Against Other Growth Factors
Other factors relevant to tumor cell proliferation, such as the intercellular adhesion molecule-1 (ICAM-1),16 have been identified as targets for mABs. Of these, bevacizumab (Avastin), which targets the vascular endothelial growth factor (VEGF), recently gained approval for the treatment of colorectal cancer. A phase II clinical trial using bevacizumab alone or in combination with chemotherapy in patients with metastatic NSCLC produced promising results.37 Other studies have investigated the use of bevacizumab: for example, in combination with the EGFR-tyrosine kinase inhibitor erlotinib (Tarceva) or as combination therapy with paclitaxel and carboplatin in the neoadjuvant setting.29,31,73 In the largest trial evaluating bevacizumab, 878 patients with recurrent or advanced NSCLC (stages IIIB and IV) were included.74 The median survival was 12.3 months in the group assigned to chemotherapy plus bevacizumab, as compared with 10.3 months in the chemotherapy-alone group. The median progression-free survival in the two groups was 6.2 and 4.5 months, respectively, with corresponding response rates of
35% and 15%. Rates of clinically significant bleeding were 4.4% and 0.7%, respectively. Based on these results, the FDA, in October 2006, granted approval for bevacizumab administered in combination with carboplatin and paclitaxel for the initial treatment of patients with unresectable, locally advanced, recurrent, or metastatic, nonsquamous NSCLC.9
35% and 15%. Rates of clinically significant bleeding were 4.4% and 0.7%, respectively. Based on these results, the FDA, in October 2006, granted approval for bevacizumab administered in combination with carboplatin and paclitaxel for the initial treatment of patients with unresectable, locally advanced, recurrent, or metastatic, nonsquamous NSCLC.9
Active Immunization with Monoclonal Antibodies (Anti-Idiotype)
In SCLC, an anti-idiotype vaccine targeting the ganglioside GD3 (BEC2) has been evaluated43. A pilot study of BEC2 plus BCG was performed in 15 patients with both limited and extensive SCLC following demonstration of a substantial response to chemotherapy. Compared with historical data, the median survival was encouraging.22 The European Organization for Research and Treatment of Cancer (EORTC) published data from a phase III trial using BEC2 in combination with induction chemoradiotherapy in limited-stage SCLC.20 A total of 515 patients were randomly assigned to receive five vaccinations of BEC2 (2.5 mg)/BCG vaccine or follow-up. The primary toxicities of vaccination were transient skin ulcerations and mild flu-like symptoms. There was no improvement in survival, progression-free survival, or quality of life in the vaccination arm. Among vaccinated patients, a trend toward prolonged survival was observed in those who developed a humoral response. The authors concluded that vaccination with BEC2/BCG has no impact on outcome of patients with limited-disease SCLC responding to combined-modality treatment. Other anti-idiotype mABs, such as 1E10,53 are currently being evaluated in early phase clinical trials.
Antibodies Linked to Cytotoxic Agents
To increase their cytocidal potency, mABs are also being linked to cytocidal agents, such as toxins, chemotherapeutic drugs, or radionuclides. Approved for clinical use are, for example, gemtuzumab ozogamicin (Mylotarg), which links the toxin calicheamicin to a CD33-specific antibody for use in the treatment of myelogenous leukemia, and ibritumomab tiuxetan (Zevalin), which links 90Y to a CD20-specific mAB. To date, the available data on comparable antibodies for the treatment of lung cancer are very limited. A phase I study investigating the immunotoxin SS1(dsFv)-PE38 in patients with advanced mesothelin-expressing malignancies was recently published, with evidence of clinical activity in a group of heavily pretreated patients.28 Other phase I trials have evaluated the murine monoclonal antibody KS1/4 linked to methotrexate in patients with NSCLC stages IIIB and IV.15 Here, six patients received KS1/4 alone and five received KS1/4-methotrexate conjugate. Mild to moderate side effects in both groups included fever, chills, anorexia, nausea, diarrhea, anemia, and brief transaminasemia. One patient who received antibody alone had an apparent acute immune complex–mediated reaction. Of the 11 patients studied, 10 had a human antimouse response. Posttreatment carcinoma biopsies revealed binding of monoclonal antibody KS1/4 and deposition of C3d and C4c complement fragments. There was one possible clinical response. Another phase I study, comprising 21 patients (18 relapsed, 3 primary refractory) with SCLC, investigated the murine mAB N901 that binds to the neural cell adhesion molecule (NCAM, CD56), which is also found on SCLC cells.45 Specific binding of the immunotoxin to tumor cells in bone marrow, liver, and lung was observed. No patient developed clinically significant neuropathy. One patient with refractory SCLC achieved a partial response. Ross and coworkers67 conducted a phase II study with 59 recurrent or metastatic NSCLC patients in order to evaluate survival, safety, efficacy, and quality of life (QOL) of treatment with SGN-15, an antibody–drug conjugate consisting of a chimeric murine monoclonal antibody recognizing the Lewis Y (Le[y]) antigen, conjugated to doxorubicin. SGN-15 plus docetaxel (compared with docetaxel alone) was well tolerated and showed some superiority in survival and QOL analyses. Other strategies consist of radioimmunoconjugates, such as the iodine 131–labeled chimeric tumor necrosis treatment, which has demonstrated some clinical efficacy in advanced NSCLC when given systemically or intratumorally.7
Other immunoconjugate-based approaches, such as the antibody-directed enzyme prodrug therapy (ADEPT) or bispecific antibodies, are currently under investigation and either have not found their way into clinical application in patients with lung cancer or, in the case of the bispecific antibody catumaxumab (anti-EpCAM x anti-CD3), are in the early stage of clinical development.77
Other Antibody-Based Strategies in the Treatment of Lung Cancer
A very interesting approach for the surgical oncologist may be the technique of radioimmuno-guided surgery (RIGS), where radiolabeled mABs are used to determine tumor-free margins.3
Most recently, data were published on a phase II trial investigating the use of mapatumumab, a fully human agonistic mAB targeting the TRAIL receptor-1, in patients with advanced NSCLC.23 The drug was well tolerated; however, none of the 32 patients treated showed an objective clinical response.
Obviously, this part of the review can only touch on the extensive body of data that is available on antibody-based immunotherapy for lung cancer. Table 121-1 lists antibodies currently under clinical investigation for lung cancer.
Tyrosine Kinase Receptor Inhibitors
Strictly speaking, tyrosine kinase (TK) inhibitors are not components of the humoral or cellular immune system. However, they are part of today’s anti-EGFR and anti-VEGFR strategies in NSCLC. Chapter 118 covers novel systemic therapy for NSCLC.
Adoptive Cell Transfer
Transfer of cellular components of the immune system has demonstrated impressive clinical response rates in metastatic melanoma.12 To date, this approach has not moved beyond an early experimental stage in lung cancer patients. Ratto and coworkers62 randomized 113 resected NSCLC patients at stages II, IIIA, and IIIB to receive either adoptive transfer of tumor-infiltrating lymphocytes (TIL) in combination with
subcutaneous rIL-2 or chemo(radio)therapy. A significant advantage in 3-year survival was observed for stage IIIB patients. A follow-up phase II study of the same group combined adoptive transfer of TIL with chemo-(cisplatin and etoposide)-radiotherapy (60 Gy) in stage III NSCLC patients. With 10 of 13 patients having progressive disease at 9 months of follow-up, the study was stopped.63 Other cell-transfer regimens consist of intravenous administration of ex vivo–generated lymphokine-activated killer (LAK) cells13,40 or lymph node lymphokine-activated killer (LN-LAK) cells.82 The application of adoptive transfer of in vitro expanded cells is limited because it exceeds the technical and financial resources of most laboratories. The available data (with low patient numbers) fail to clearly demonstrate clinical benefit from this approach. However, the adoptive transfer of tumor-specific T cells in patients pretreated with nonmyeloablative chemotherapy, a strategy that is highly effective in metastatic melanoma,12 has yet to be studied in NSCLC patients.
subcutaneous rIL-2 or chemo(radio)therapy. A significant advantage in 3-year survival was observed for stage IIIB patients. A follow-up phase II study of the same group combined adoptive transfer of TIL with chemo-(cisplatin and etoposide)-radiotherapy (60 Gy) in stage III NSCLC patients. With 10 of 13 patients having progressive disease at 9 months of follow-up, the study was stopped.63 Other cell-transfer regimens consist of intravenous administration of ex vivo–generated lymphokine-activated killer (LAK) cells13,40 or lymph node lymphokine-activated killer (LN-LAK) cells.82 The application of adoptive transfer of in vitro expanded cells is limited because it exceeds the technical and financial resources of most laboratories. The available data (with low patient numbers) fail to clearly demonstrate clinical benefit from this approach. However, the adoptive transfer of tumor-specific T cells in patients pretreated with nonmyeloablative chemotherapy, a strategy that is highly effective in metastatic melanoma,12 has yet to be studied in NSCLC patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


