Chapter 5 Normal Mechanisms of Vascular Hemostasis
Endothelial Function and Platelet Activation
Platelets are anucleate cells produced by megakaryocytes in the bone marrow. Once they have traversed from the bone marrow to the general circulation, their lifespan is approximately 10 days. They function mainly to limit hemorrhage after trauma resulting in vascular injury. Normally in the vasculature, platelets are in a resting state and only become activated after exposure to a stimulus leads to a shape change and release reaction that causes the platelet to export many of its biologically important proteins. Some of the agonists that can initiate this response include thromboxane A2, adenosine diphosphate (ADP), thrombin, and serotonin. In areas of vascular injury, platelets are attracted to the impaired site by collagen through binding with von Willebrand factor (vWF) via the glycoprotein (GP) Ib/V/IX complex. This initial binding results in platelet activation, with a subsequent feedback mechanism in which ADP, thrombin, and thromboxane A2 further activate the platelets and recruit additional platelets to the area. The complex firmly binds the platelet to the area of injury so there is no disruption by the high shear forces of turbulent blood flow that occur with vessel disruption. This amplification of the response is essential to form a hemostatic plug and represents the first stage in the hemostatic process. When vWF is not present, hemostatic abnormalities result, with deficiencies leading to von Willebrand’s disease, which can be associated with severe bleeding. Hemostasis issues also arise when the platelet receptor complex GPIb/V/IX is mutated, resulting in inability of vWF to bind, a disorder termed Bernard-Soulier’s syndrome.1,2
Additional platelet aggregation occurs through activation of G protein–coupled receptors (GPCRs), with the final pathway relying on the GP IIb/IIIa complex, the main receptor for platelet aggregation and adhesion.3,4 Fibrinogen tethers GP IIb/IIIa complexes on different platelets, stabilizing the clot. The integral role of this receptor is manifest in Glanzmann thrombasthenia, a disorder in which fibrinogen binding is impaired, leading to spontaneously occurring mucocutaneous bleeding episodes.5
Vascular endothelium is essential to this hemostatic process; this is the cellular site where regulation and initiation of coagulation begins. Endothelial cells (ECs) modulate vascular tone, generate mediators of inflammation, and provide a resistant surface that allows for platelets to experience laminar flow with minimal shear. Endothelial cells regulate hemostasis by releasing a number of inhibitors of platelets and inflammation. Vascular endothelium is essential for regulating uncontrolled platelet activity through mechanisms of inhibition including the arachidonic acid–prostacyclin pathway, L-arginine–nitric oxide pathway, and endothelial ectoadenosine diphosphatase (ecto-ADPase) pathway6 (Table 5-1).
Table 5-1 Factors Involved in Fibrinolysis
| Prohemostatic | Antihemostatic |
|---|---|
| Circulating | |
| α2-Antiplasmin | Antithrombin III |
| Thrombin | Protein C |
| Thrombin-activatable fibrinolysis inhibitor (TAFI) | Protein S |
| Tissue factor pathway inhibitor (TFPI) | |
| Endothelium-Derived | |
| Plasminogen activator inhibitor-1 (PAI-1) | Ectoadenosine diphosphatase (Ecto-ADPase)/CD39 |
| Tissue factor (TF) | Heparan sulfate (HS) |
| von Willebrand factor (vWF) | Nitric oxide (NO) |
| Thrombomodulin | |
| Tissue plasminogen activator (tPA) | |
| Urokinase plasminogen activator (uPA) | |
Nitric oxide (NO) is produced constitutively by (ECs) via an endothelial isoform of nitric oxide synthase (eNOS) in a process dependent on conversion of L-arginine to L-citrulline. Vascular tone is regulated by NO as it controls smooth muscle cell (SMC) contraction. It also inhibits platelets directly, blocking platelet aggregation through stimulation of guanylyl cyclase and cyclic guanosine monophosphate (cGMP) and inhibition of platelet phosphoinositol3-kinase (PI-3 kinase). Nitric oxide functions by decreasing the intracellular Ca2 + level through cGMP, which inhibits the conformational change in GP IIb/IIIa suppressing fibrinogen’s ability to bind to the receptor, thereby attenuating platelet aggregation.7
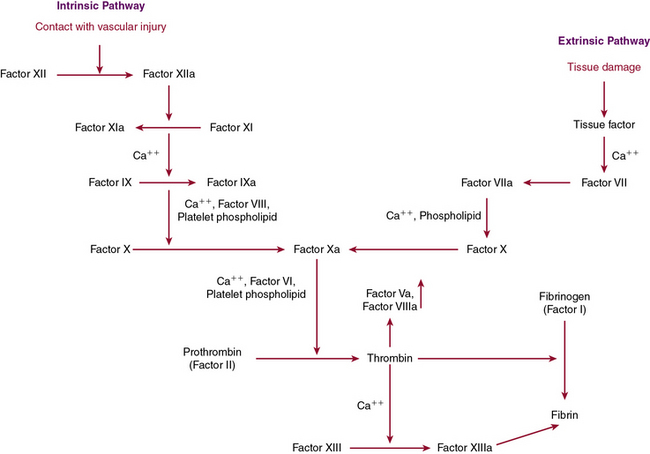
The last pathway important in modulating vascular endothelium’s interaction with platelets is the endothelial ecto-ADPase pathway, which impairs ADP-mediated platelet activation. By hydrolyzing ADP, this enzyme inhibits the critical state of platelet recruitment to a growing aggregate, thereby limiting thrombus formation. Once the platelet aggregate has been stabilized by fibrin with red cells to the vessel wall, the next stage of hemostasis involves activation of the highly regulated coagulation cascade (Fig. 5-1).
Coagulation Cascade Leading to Fibrin Formation
Disruption in the endothelium not only recruits platelets for plug formation, it also stimulates activation of the coagulation cascade, which is essential for secondary clot formation through fibrin generation. The coagulation cascade is a dynamic integrated process in which each step is dependent on another step for activation of proenzymes or zymogens to their active forms through proteolytic cleavage. This process is dependent upon calcium and the phospholipid bilayer allowing inactive clotting factors to be converted to active enzymes through serine protease activity. These coagulation proteins function in a step-by-step fashion to activate downstream members of the cascade, leading to production of the penultimate clotting factor, thrombin. Thrombin is versatile, playing a role in many of the essential stages of hemostasis. Not only is it important for platelet activation, it is also necessary for the cross-linking of fibrin. Recently there have been attempts to limit thrombus formation by directly inhibiting thrombin activity through anticoagulants such as ximelagatran and the oral medication, dabigatran, which is now available for clinical use.8
The clotting cascade is divided into two main pathways, the intrinsic and extrinsic pathways. The extrinsic pathway begins with establishment of a complex between tissue factor, found on the cell surface or on microparticles, and factor VIIa. This complex leads to activation of factor X to Xa, which can then further the response by looping back and converting factor VII to VIIa in a feedback mechanism. When factor Xa is present, it binds to factor Va on the membrane surface and again generates prothrombinase, which converts prothrombin to thrombin and then generates fibrin as detailed earlier. The activity of factor Xa is accelerated by the presence of factor Va through calcium and formation of a noncovalent association γ-carboxyglutamate residues of factor Xa and the phospholipid surface of activated platelets.9 The extrinsic pathway is measured by prothrombin time (PT), which is determined by adding an extrinsic substance such as tissue factor or thromboplastin.10
The extrinsic pathway, which is dependent on tissue factor, appears to be the main pathway responsible for hemostasis, with the intrinsic pathway playing a supporting role. Tissue factor is a membrane-bound GP that is constitutively expressed by SMCs and fibroblasts but selectively expressed by ECs when there is vessel wall injury. The “encrypted” activated form of factor VIIa is made functional through a conformational change that occurs at cysteines 186 and 209, leading to disulfide bond formation upon vessel wall injury. Protein disulfide isomerase, glutathione, and NO all may have a role in these allosteric changes; however, recent studies have questioned the importance of “de-encryption” in this process.11–14 Tissue factor functions through activation of factors X and IX after interactions with factor VII as a complex. Factor VII, although at low levels in an active state (factor VIIa) in the circulation, only becomes biologically important after it is bound to tissue factor in complex with factors X and IX. This complex formation is essential for activation of thrombin.9
The role of tissue factor has recently been expanded. It circulates in the blood in association with microvesicles that are derived from cellular membranes produced from lipid rafts on monocytes and macrophages.15 These tissue factor–bearing microvesicles can directly initiate the coagulation cascade on activated platelets in a process that may be important for understanding the hypercoagulable state.16,17
The extrinsic pathway, described earlier, joins up with the intrinsic pathway through factor X to form the common pathway. The intrinsic pathway is initiated by contact and results in activation of factor IXa, which then goes on to activate factor X as described. It is generally accepted that the intrinsic pathway is of less importance in coagulation than the tissue factor–mediated extrinsic pathway, although it plays an essential role in inflammation and fibrinolysis. The intrinsic pathway is based on exposure of blood to a negatively charged surface, and is classically initiated by activation of factor XIIa by kallikrein, which is facilitated by kininogen. Kallikrein is generated from prekallikrein through proteolytic cleavage by activated factor XII in a reaction dependent on the presence of high-molecular-weight kininogen (HMWK). When kallikrein has been generated, it also functions to cleave HMWK to bradykinin, which functions as an inflammatory mediator to potentiate vasodilation and vascular permeability, thereby expanding the role of factor XIIa to inflammation, regulation of vascular tone, and fibrinolysis.18 Activated factor XII catalyzes conversion of factor XI to the active enzyme form, factor XIa. When calcium is present, factor XIa next functions to convert IX to IXa, which then binds to VIIIa on membrane surfaces, converting X to its active form, factor Xa. Factor Xa then binds to Va on the membrane surface to generate prothrombinase, which converts prothrombin to thrombin. As thrombin is formed, two small prothrombin fragments, termed molecules F1 and F2, are released and can be used as markers of serum thrombin formation.19 The intrinsic pathway is monitored through the activated partial thromboplastin time (APTT), which relies on foreign substances such as glass or silicates to activate factor XII to initiate the pathway. Deficiencies in the earliest states of the intrinsic pathway, when prekallikrein, HMWK, and factor XII are involved, are not associated with bleeding tendencies and therefore do not lead to a bleeding diathesis, even though there is an elevation in partial thromboplastin time. Mutations in factor XII have been reported in a group of patients with hereditary angioedema, although there does not appear to be a bleeding diathesis with this disorder. Some initial studies have suggested that factor XII polymorphisms may be associated with an increased propensity for thrombosis, but this has not been validated.20,21
When factor Xa generates thrombin, the intrinsic and extrinsic pathways have merged into the common pathway. Thrombin is essential for fibrinogen to generate fibrin, which is released through proteolytic cleavage.22 The fibrin molecules that are generated have polymerization sites exposed, making it easier for fibrin to cross-link noncovalently. This cross-linking enables platelets to be entrapped in a meshwork of fibrin strands to form the secondary clot through the action of factor XIII, activated by thrombin.23 In the process of cross-linking, there is also an inherent mechanism of autoregulation, with the binding sites necessary to initiate fibrinolysis being blocked so the clot does not self-destruct.
Antithrombin is a serine protease inhibitor that binds specifically to factors IXa, Xa, and thrombin, thereby inactivating them. Antithrombin has two main binding sites that maintain its functionality: the reactive center at Arg 393/Ser 394 and the heparin binding site at the amino-terminal end of the molecule. Binding of both endogenous and exogenous heparins at this site causes a conformational change in antithrombin that enables it to inactivate its targets at an accelerated rate. The glycosaminoglycan heparan sulfate (HS), present on the surface of ECs, mediates antithrombin’s ability to increase its activity and functions as the physiological equivalent to heparin.24 Deficiency of antithrombin is associated with a genetic propensity to form venous thrombosis, discussed in Chapter 10.25
Activated protein C (APC) and protein S are also important mechanisms for preventing excessive clotting. During the clotting process, thrombin binds to thrombomodulin, which is also present on the EC surface. It then undergoes a conformational change leading to activation of protein C.26 Activated protein C complexes with protein S and proteolytically cleaves factors Va and VIIIa, resulting in their inactivation and a decrease in generation of factors Xa and thrombin. Cleavage of factor Va occurs at Arg 506, Arg 306, and Arg 679 by APC in a sequential manner such that the cleavage at Arg 506 exposes cleavage sites at the other sites through a conformational change. Mutation of the arginine located at position 506 to glutamine leads to factor V Leiden, which is associated with a hypercoagulable state.