Fig. 33.1
Plethysmographic (PVR) tracings at thigh, calf, and toe levels in a 4-year-old girl with multiple congenital AV fistulas involving the entire left leg (Reprinted from Rutherford [5]. With permission from Elsevier)
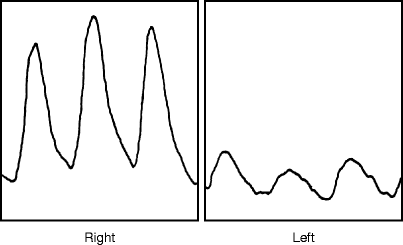
Fig. 33.2
Toe plethysmographic tracings from a patient with a congenital AVM involving the left calf. The reduced PVRs reflect a distal steal from this, but not to critical ischemic levels for some pulsitility remains. The left ankle pressures was 55 mmHg (Reprinted from Rutherford [5]. With permission from Elsevier)
Velocity Waveform Analysis
Velocity tracings can be recorded over any extremity artery by a Doppler probe connected to the DC recorder and strip chart, or more commonly nowadays, by the velocity readout of a duplex scanner. This is increasingly being done by the latter technique nowadays, not only because the duplex scan has become the “workhouse” of most VDLs but also because it offers other valuable information in this setting (see below). The strip chart recording of velocity waveforms generated by a Doppler probe is uncommonly performed today as a separate test. Nevertheless, the characteristic findings are the same with either instrument and will be described here. In evaluating for AVFs, the velocity is recorded over the major proximal inflow artery, e.g., femoral or axillary. The reason for selecting this location, rather than directly over the suspected fistula(s) will become apparent later, in describing duplex scan findings. A high-velocity flow pattern in an artery leading to the area of suspicion is good evidence that the artery is serving as the inflow for AVFs [7, 8]. For many if not most clinical purposes, a qualitative estimate of flow velocity and the contour of the analog velocity tracings or “waveforms” obtained in this manner with a directional Doppler velocity detector, or duplex scanner, provides sufficient information for diagnosing the presence of AVF(s), and the magnitude of the changes provides some indication of the magnitude of fistulous flow.
To recognize a tracing diagnostic of AVFs, one must realize that, in contrast, the velocity tracings of a resting normal extremity is characterized by end-systolic reversal following peak systolic flow, followed by low flow in early diastole and negligible flow in late diastole. Such a low-flow, high-resistance pattern is most pronounced in the lower extremity. In the upper extremity, there may be little end-systolic reversal. However, high-flow low-resistance arterial velocity pattern, while seen in a number of high flow visceral arteries (e.g., the renal, carotid, celiac arteries), in the extremities, such a high-flow pattern is seen after exercise, and after sympathetic blockade, but, importantly, also in association with AVFs. In these settings, peak systolic velocity may be quite high but, of more diagnostic significance, there is continuous flow throughout diastole and a the “dip” in the tracing between systole and diastole does not approach the zero velocity baseline, let alone show the typical end-systolic reversal of the normal resting extremity. The characteristic arterial pattern associated with arteriovenous fistulas, as shown in Fig. 33.3, thus consists of (1) an elimination of end-systolic reversal and (2) a marked increase in diastolic velocity, which appears to “elevate” the entire tracing above the zero-velocity baseline. The degree of elevation in end-diastolic velocity correlates directly with the flow increase caused by the arteriovenous fistula [5, 6]. By using these characteristic Doppler velocity signals as a guide, one can detect and localize congenital arteriovenous communications that otherwise might escape detection [9, 10]. Peripheral AVFs constituting 5% of extremity flow or more can be readily detected by this means alone. This test is more sensitive than segmental pressures and plethysmography, and can be detected by a duplex scan.
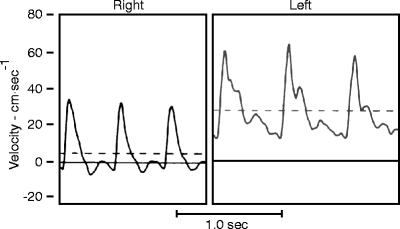

Fig. 33.3
Velocity tracing from the femoral arteries in a 4-year-old girl with a large AVM involving the left thigh. Note that the tracing on the left, compared to the normal right tracing, has higher peak and mean (dashed line) systolic velocity, and there is no end systolic reversal. Rather, there is high flow continuing throughout diastole, as a result of which the tracing does not drop back to the zero baseline at the end of diastole, but is elevated well above the zero baseline (Reprinted from Rutherford [5]. With permission from Elsevier)
Although these changes are diagnostic enough that comparison with the other extremity would not seem necessary, this comparison still holds value, to rule out hyperemia and similar velocity signals there. Hyperdynamic flow is associated with conditions such as beriberi or thyrotoxicosis but since, in these conditions, the effect is generalized, it would affect all extremities, emphasizing the importance of comparing with the contralateral normal extremity. False-positives can occur in other hyperemic settings, e.g., inflammation associated with superficial thrombophlebitis, lymphangitis, bacterial infection, and thermal or mechanical trauma. Other causes of hyperemia isolated to an individual vessel or limb (e.g., exercise or reactive hyperemia following a period of ischemia) are transient. Externally applied heat, local infection (e.g., cellulitis or abscess), or sympathetic blockade (permanent or transient, as in epidural anesthesia) can also increase flow velocity and give this pattern, but none of these should create any significant confusion in the usual patient referred to the VDL for evaluation of congenital AVFs, and some (e.g., throtoxicosis, beri-beri, high fever) should produce bilaterally equal changes.
Evaluation for Congenital Arteriovenous Fistulas Using These Three Physiologic Tests in Combination
These three tests are preferably done in combination for their findings and reinforce each other, and they share the advantages that they are inexpensive, easily applied, and require only basic operator or interpretive skill. The instrumentation is simple and used on an everyday basis in most VDLs. Some limitations to the above tests must be noted, however. They include the following: (1) These tests give qualitative rather than quantitative information. (2) They can be applied only to arteriovenous fistulas located in the extremity proper (i.e., at or below the highest cuff or point of Doppler probe interrogation). Thus, pelvic AVMs could not be studied by these methods. (3) It should again be noted that in children appropriately smaller cuffs are required. (4) These tests may not detect diffuse congenital micro fistulas or overall fistula flow constituting less than 5% of total extremity flow. (5) Single limb studies may not be diagnostic unless compared with a normal contralateral extremity. Nevertheless, in combination, these tests can be very useful for screening for congenital AVFs in the extremities of patients presenting with suggestive signs (e.g., a vascular “birthmark,” atypical (early onset/unusual location) varicose veins, or limb enlargement), and for detecting, roughly localizing, and assessing the relative magnitude of such congenital lesions. With anatomically localized lesions, these tests, with or without duplex scanning (see below) suffice for most clinical decision-making.
Duplex Scanning
It is recognized that duplex ultrasound scanning plays an increasing role in today’s VDL. The basic duplex scanner combines an ultrasound image with a focused directional Doppler probe. In modern instruments, the velocity signal is color-coded so that red represents arterial flow and blue represents venous flow (going in opposite directions). The velocities are also displayed on the screen, as needed, for specific applications (e.g., bypass graft surveillance, carotid artery interrogation).
Because the duplex scanner provides velocity information, it can serve as a practical means of performing velocity waveform analysis, the observed patterns serving as a simple yet sensitive means of diagnosing an AVF. Because of the other additional information obtainable from duplex scanning, it has mostly replaced using a simple Doppler probe connected to a DC recorder and strip chart for this purpose. High-peak mean velocity readings recorded over the main inflow artery of the involved extremity, compared with those at the same location of the contralateral normal extremity, will often confirm the presence of an arteriovenous fistula in that limb. As pointed out above, the characteristic pattern of the velocity tracing, with its elevated end-diastolic flow, and its extended persistence over the inflow artery should distinguish this high-velocity reading from the more focused high-velocity reading observed in association with a arterial or bypass graft stenosis.
The software of some of today’s duplex scanners also allows a rough estimation of volume flow, with diameter measurements being used to estimate cross-sectional area and the velocity signals and the angle of incidence of the probe allowing the Doppler equation to be applied (flow = velocity (frequency shift) × cosine theta (angle of incidence of the ultrasound beam) × cross-sectional area, divided by C (velocity of sound in tissue, a constant)). However, a problem in using the duplex scan to obtain accurate velocity or flow measurements directly over an AVF is the presence of turbulence and multidirectional flows associated with aliasing. On the other hand, the flashes of yellow representing turbulent fistula flow will be seen, and these along with higher than normal arterial velocities upstream are, in themselves, diagnostic of AV shunt flow. So the diagnosis is readily made by this approach, but quantification is not possible at the fistula site. Congenital arteriovenous fistulas are more complex, especially when part of an AVM, but their high-flow patterns are readily recognized and, by moving the scan head, the nature and extent of the more localized superficial lesions can sometimes be delineated. This in itself can be diagnostic, and is particularly useful when applied to mass lesions, which often present with a network of varicosities near the surface of the skin. Higher than normal flows in these veins will also betray an underlying AVM. The diagnostic dilemma here is that these varicosities may either be part of a venous malformation or be associated with an underlying arteriovenous malformation, but this be resolved by their flow characteristics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


