The aim of this study was to analyze using noninvasive cardiac examinations a series of young athletes discovered to have ventricular arrhythmias (VAs) during the preparticipation screening program for competitive sports. One hundred forty-five athletes (mean age 17 ± 5 years) were evaluated. The study protocol included electrocardiography (ECG), exercise testing, 2-dimensional and Doppler echocardiography, 24-hour Holter monitoring, signal-averaged ECG, and in selected cases contrast-enhanced cardiac magnetic resonance imaging. Results of ECG were normal in most athletes (85%). VAs were initially detected prevalently during exercise testing (85%) and in the remaining cases on ECG and Holter monitoring. Premature ventricular complexes disappeared during exercise in 56% of subjects. Premature ventricular complexes during Holter monitoring averaged 4,700 per day, predominantly monomorphic (88%), single, and/or in couplets (79%). The most important echocardiographic findings were mitral valve prolapse in 29 patients (20%), congenital heart disease in 4 (3%), and right ventricular regional kinetic abnormalities in 5 (3.5%). On cardiac magnetic resonance imaging, right ventricular regional kinetic abnormalities were detected in 9 of 30 athletes and were diagnostic of arrhythmogenic right ventricular cardiomyopathy in only 1 athlete. Overall, 30% of athletes were judged to have potentially dangerous VAs. In asymptomatic athletes with prevalently normal ECG, most VAs can be identified by adding an exercise test during preparticipation screening. In conclusion, cardiac screening with noninvasive examinations remains a fundamental tool for the identification of a possible pathologic substrate and for the characterization of electrical instability.
Athlete’s heart is generally regarded as a benign electroanatomic remodeling due to systematic training. Nevertheless, repolarization inhomogeneity of myocardium, a high prevalence of ventricular arrhythmias (VAs), and arrhythmogenic right ventricular (RV) cardiomyopathy–like phenotypes have been described in some athletes. Sports are associated with an increased risk for sudden death in athletes who are affected by cardiovascular conditions predisposing to life-threatening VAs during exercise. The incidence of sudden cardiac death in young competitive athletes has substantially decreased in the Veneto region of Italy thanks to the introduction of a preparticipation screening program that identifies subjects with previously unrecognized cardiovascular conditions. Rhythm and conduction abnormalities are the first cardiovascular causes of sports disqualification, and evaluation of VAs constitutes an important medical and legal issue. The aim of this study was to analyze using noninvasive cardiac examinations a series of young athletes discovered to have VAs.
Methods
One hundred forty-five young, nonelite, competitive athletes (mean age 17.3 ± 5.3 years, range 9 to 34; male/female ratio 106/39 = 2.7) were evaluated in our laboratory during a period of 3 years. All subjects were referred because of VAs detected during preparticipation screening, which also included exercise testing. The study protocol included family and personal histories, 12-lead electrocardiography (ECG), 2-dimensional echocardiography with Doppler analysis, 24-hour Holter monitoring, exercise testing, signal-averaged ECG, and in selected cases contrast-enhanced cardiac magnetic resonance imaging.
Electrocardiograms were evaluated using digital calipers at standard paper speed (25 mm/s). Electrocardiographic abnormalities were divided into 2 groups (common or training-related and uncommon or training-unrelated abnormalities) and interpreted considering the most recent recommendations. Signal-averaged ECG was performed using a MAC15 system (Marquette Inc., Milwaukee, Wisconsin). The following parameters for each of the 3 filters (25, 40, and 80 to 250 Hz) were evaluated: filtered QRS duration, high-frequency low-amplitude signal duration in the terminal portion of the filtered QRS interval with a voltage amplitude <40 μV (or <20 μV for the 80- to 250-Hz filter), and the root mean square of the voltage in the last 40 ms of the filtered QRS interval. Presence of late potentials was considered when ≥2 parameters were abnormal in 1 filter. The exercise test was performed on a bicycle or treadmill (with standard 12-lead placement) up to the submaximal heart rate, calculated from the formula 220 − age × 85%. ST-segment alterations and VAs were carefully evaluated. Holter monitoring was performed using 12-lead ECG (with standard lead placement). The number, morphologies, and coupling intervals of single and repetitive VAs were studied.
The echocardiographic study was performed with a 2.5- to 4-MHz transducer (model 5500, Philips Medical Systems, Andover, Massachusetts) and included M-mode, 2-dimensional, and Doppler examinations of the traditional views. Left ventricular (LV) end-diastolic diameter, parietal wall thickness, and left atrial diameter were calculated in the parasternal long-axis view using M-mode imaging. LV end-diastolic volume, end-systolic volume, and ejection fraction were calculated in the apical 4-chamber view (using Simpson’s rule). RV end-diastolic area, end-systolic area, and fractional area change were calculated from the apical 4-chamber view, and the RV ejection fraction was also measured. The RV outflow tract was measured in the parasternal view and the short-axis view and the RV inflow tract in the 4-chamber view. The presence of LV and RV wall motion abnormalities was assessed.
Cardiac magnetic resonance imaging was performed using a 1.0-T clinical scanner (Harmony; Siemens Healthcare, Erlangen, Germany) using a phased-array cardiac receiver coil. After the intravenous administration of gadolinium, chelate inversion recovery prepared, breath-hold cine gradient-echo images were obtained. Cine, morphologic, and late gadolinium enhancement images acquired during the same imaging session were matched by slice position.
Data are expressed as mean ± SD for continuous variables and as frequencies with percentages for categorical variables. All continuous variables are expresses as mean ± SD.
Results
The initial detection of premature ventricular complexes (PVCs) during the preparticipation program was due mostly to the exercise tests in 124 athletes (85%). In the remaining cases, PVCs were present on ECG (7 athletes [5%]) or detected on Holter monitoring (14 athletes [10%]).
Baseline electrocardiographic results were normal in 123 athletes (85%) (in 90 [62%] with the common abnormalities) and abnormal in 22 (15%) with the uncommon abnormalities. Mean values of rhythm (69 ± 11 beats/min), electrical QRS axis (73 ± 2°), PQ interval (147 ± 22 ms), QRS duration (93 ± 14 ms), corrected QT interval (414 ± 19 ms), and isolated QRS voltage criteria for LV hypertrophy (SV 1 + RV 5 /V 6 = 27 ± 8 mV) were normal. All athletes were in sinus rhythm. A mild right axis deviation of the QRS complex (+105°), present in 15 athletes (10.3%), was considered normal. The electrocardiographic common abnormalities were sinus bradycardia in 28 athletes (19.3%), first-degree atrioventricular block in 4 (2.8%), incomplete right bundle branch block (RBBB) in 37 (25.5%), isolated increases in QRS voltage in 17 (11.7%), and early repolarization in 50 (34.5%). The electrocardiographic uncommon abnormalities were left or right atrial enlargement in 4 athletes (2.8%), ventricular preexcitation in 1 (0.7%), complete RBBB in 3 (2.1%), signs of RV hypertrophy in 2 (1.4%), nondiagnostic Brugada-like ST-segment abnormalities in 4 (2.8%), ST-segment depression in 2 (1.4%), T-wave inversions in 8 (5.5%), and corrected QT interval prolongation in 3 (2.1%; 450 to 460 ms, male athletes). Normal T waves were present in 137 athletes (94.5%). Negative T waves in lead V 1 were present in 99 athletes (68.2%) but were rare in other precordial leads: V 1 to V 2 (2.1%; age ≤14 years), V 1 to V 3 (1.4%), V 1 to V 4 (0.7%), lateral leads (0.7%), and ≥2 inferior leads (1.4%). Intraventricular conduction delays with QRS durations >120 ms were present in 4 athletes always, in conjunction with negative T waves in >1 precordial or inferior lead. VPCs were present on ECG in 33 athletes (22.7%).
Signal-averaged ECG was performed in 129 athletes (89%). Ten (6.9%) showed late potentials: 6 in 1 filter and 4 in 2 filters. The mean values of each filter were normal: filtered QRS duration 120.1 ± 11.4, 110.9 ± 14.8, and 97.1 ± 13 ms; high-frequency low-amplitude signal duration 17.8 ± 10.4, 28.8 ± 26.9, and 25.1 ± 11.3 ms; and root mean square of the voltage in the last 40 ms of the filtered QRS interval 105.6 ± 58.3, 50.5 ± 22.3, and 34.7 ± 23.6 mV.
Concerning echocardiographic alterations, typical findings of athlete’s heart were detected in 38 subjects (26%; Table 1 ). In detail, LV end-diastolic diameter was increased (>56 mm) in 10% (mean 50 ± 5 mm), and ejection fractions were normal (mean 61 ± 4%) in all but 1. None had a parietal wall thickness >11 mm (septal wall 8 ± 1.2 mm, posterior wall 7.8 ± 1.2 mm). E/A ratios were normal in all (E = 82 ± 13 cm/s, A = 44 ± 12 cm/s) but 1 subject. Ea/Aa ratios, assessed in 68 athletes, were normal (Ea = 19.4 ± 4.8 cm/s, Aa = 7.9 ± 2.3 cm/s, Sa = 11.5 ± 2.9 cm/s). Left or right atrial enlargement was present in 10 athletes (6.9%). The presence of ≥1 LV false tendon was detected in 22%. RV enlargement (RV end-diastolic area >24 cm 2 ) was present in 17% (mean 21 ± 4 cm 2 ). The RV inflow tract was increased in size (>41 mm) in 19%. The RV outflow tract in the parasternal view was increased in size (≥19 mm/m 2 ) in 3 athletes, and the RV outflow tract in the short-axis view was increased in size (≥21 mm/m 2 ) in 2 athletes. RV systolic function was normal (fractional area change 45.5 ± 5.5%, ejection fraction 61.1 ± 4.6%) in all but 1 subject. Rich trabeculation (29%), globular-shaped apex (16%), and hyperechogenic moderator band (16%) were quite common. RV Ea/Aa ratios, assessed in 63 athletes, were normal in all (Ea = 14.9 ± 3.3 cm/s, Aa = 8.2 ± 2.9 cm/s, Sa = 13.1 ± 2.6 cm/s). Trivial regurgitations were detected in the tricuspid valve (75%), the mitral valve (63%), the pulmonary valve (41%), and the aortic valve (11%). One athlete had mild pulmonary hypertension. Mitral valve prolapse was found in 29 athletes (20%; 4 with mild regurgitation). Congenital heart disease was detected in 4 athletes (2.8%; a bicuspid aortic valve, a ventricular septal defect, a partial anomalous pulmonary venous return, and a persistent left superior vena cava). The suspicion of arrhythmogenic RV cardiomyopathy was raised in 3 subjects (2.1%), and isolated RV apical hypokinesia was found in another 2 (1.4%).
| Finding | n (%) |
|---|---|
| Specific findings (n = 36) | |
| Mitral valve prolapse | 29 (20%) |
| Congenital heart diseases ∗ | 4 (2.7%) |
| Suspected arrhythmogenic RV cardiomyopathy | 3 (2.1%) |
| Nonspecific findings (n = 72) † | |
| Atrial septal aneurysm | 3 (2.1%) |
| Apical RV hypokinesia | 2 (1.4%) |
| Pericardial effusion | 1 (0.7%) |
| LV false tendon (≥1) | 32 (22%) |
| RV enlargement | 11 (7.6%) |
| LV enlargement | 9 (6.2%) |
| Biventricular enlargement | 6 (4.1%) |
| Mild pulmonary regurgitation | 12 (8.4%) |
| Mild tricuspid regurgitation | 8 (5.5%) |
| Mild mitral regurgitation without prolapse | 3 (2.1%) |
| Mild aortic regurgitation | 2 (1.4%) |
| Isolated left papillary muscle hypertrophy | 1 (0.7%) |
∗ Bicuspid aortic valve with mild valvular stenosis, ventricular septal defect, partial anomalous pulmonary venous return, and persistent left superior vena cava; a Wolff-Parkinson-White electrocardiographic pattern was also found.
† More than 1 nonspecific finding was present in some subjects.
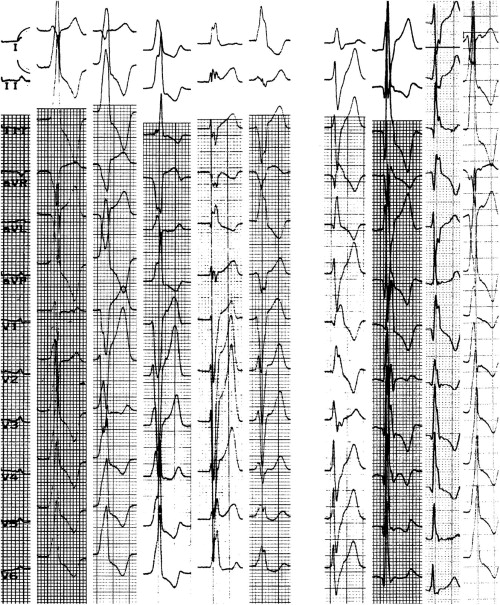
Holter monitoring showed a mean number of PVCs of 4,700 per day. The number of PVCs ranged from 0 to 720 per day in 49 athletes (33.8%), from 720 to 5,000 per day in 46 (31.7%), from 5,000 to 10,000 per day in 29 (20%), and from 10,000 to 20,000 per day in 17 (11.7%) and was >20,000 per day in 4 (2.8%). In 26 subjects (18%), PVCs numbered <20 per day and were judged nonsignificant. Only single PVCs were detected in 83 athletes (57%), and in the remaining athletes, repetitive forms were also detected (43%). Single PVCs had in 98% of subjects wide coupling intervals (>400 ms, mean 506 ± 104 ms). Among the 119 athletes with significant numbers of PVCs, in 105 (88%), PVCs were monomorphic. The most frequent PVC morphologies were left bundle branch block (LBBB) with inferior axis deviation (IAD) in 59 subjects (50%), RBBB with left axis deviation (LAD) in 21 (18%), LBBB with LAD in 18 (15%), RBBB with right axis deviation in 14 (12%), LBBB with normal axis in 14 (12%), and RBBB with IAD in 8 (7%) ( Figure 1 ). IAD was considered between +75° and +105° and LAD ≤−30°. A total of 44 athletes (30%) showed ventricular couplets (monomorphic in 32, polymorphic in 12), with a mean coupling interval of 457 ± 181 (<400 ms in 11 [24%]); in 5 subjects, ventricular couplets were frequent (>100 per day), and in 1 case, they were frequent and short coupled. Asymptomatic, nonsustained ventricular tachycardia (VT) was present in 31 athletes (21%). Of these, 27 showed brief VT (3 to 10 beats): 17 presented triplets (mean rate 138 beats/min) and 10 presented runs from 4 to 10 beats (mean rate 170 beats/min). In 4 athletes, VT episodes of >10 beats were observed. Most athletes (n = 22 [71%]) showed single runs of VT during the day. The mean ventricular rate of all VTs was 130 beats/min (mean R-R interval 461 ms): 4 VTs (13%) with rates >210 beat/min, 9 (33%) with rates of 150 to 210 beats/min, 11 (35%) with rates of 100 to 150 beats/min, and 7 (22.5%) with rates <100 beats/min. Moreover, 4 athletes presented short runs of polymorphic VT. Overall, 13 VTs (9%) were judged potentially dangerous on the basis of electrocardiographic characteristics (short R-R interval, multiple or long episodes, polymorphism, exercise induction) or the presence of an organic substrate.
Submaximal exercise tests were performed in 138 athletes (95%), and in 124 (90%), PVCs were recorded. Four basic patterns of PVC response to effort were found: (1) PVCs that disappeared during effort, reappearing during the recovery phase in 77 athletes (55.8%), (2) PVCs that appeared only during the recovery phase in 16 (11.6%), (3) PVCs that persisted during all exercise in 12 (8.7%), and (4) exercise-induced PVCs in 19 (13.8%) (3 with repetitive forms; in 15 cases, PVCs were totally absent at rest during Holter monitoring). Effort-induced PVCs showed LBBB morphology with IAD in 7 athletes (37%), RBBB with LAD in 6 (31%), LBBB with LAD in 3 (16%), LBBB with normal axis in 1 (0.7%), and RBBB with right axis deviation in 1 (0.7%). In 1 athlete, polymorphic PVCs were present. Supraventricular arrhythmias were present in 3.6%. No significant ST-T changes were recorded.
Thirty athletes underwent contrast-enhanced cardiac magnetic resonance imaging. Results were normal in 14; in 3, results confirmed a left superior vena cava, a partial anomalous pulmonary venous return, and a hypertrophic papillary muscle, and in 1 subject, mild septal late enhancement was present. Twelve athletes had ≥1 RV abnormal finding: 1 with moderate RV enlargement and 2 with mild diffuse hypokinesia. In 9 athletes, the presence of regional wall motion abnormalities was detected: apical hypokinesia in 3, RV systolic bulging in 3, akinetic regions in 2, and a dyskinetic region in 1. Among these subjects, 2 also showed late enhancement, and 3 showed regional fatty infiltrations. Only 1 subject presented with regional dysfunction plus a structural alteration (1 minor criterion for arrhythmogenic RV cardiomyopathy diagnosis).
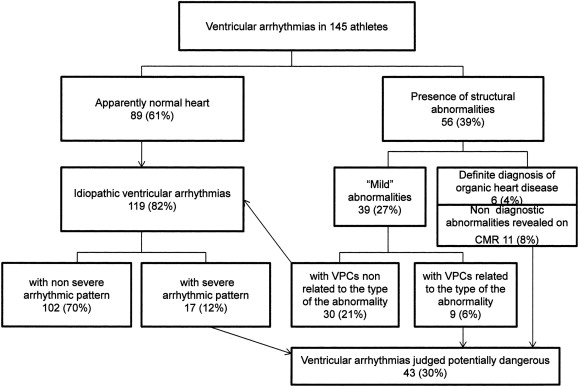
After clinical evaluation, 30% of athletes were judged to have potentially dangerous VAs on the basis of the presence of a morphologic substrate and/or the characteristics of VA pattern according to the recommendations for competitive sports, tailored to each athlete and type of sport ( Figure 2 ). Borderline cases belonging to the “gray zone” of diagnosis between athlete’s heart and arrhythmogenic RV cardiomyopathy were also judged potentially dangerous. Among these athletes, 10% (n = 14) were treated with antiarrhythmic drugs, in 1.4% (n = 2) ablation was indicated, and in 1 athlete surgical repair of the congenital defect was indicated. In the rest of the athletes, competitive sports were not recommended, and detraining was proposed (n = 26). Follow-up was feasible in 93 athletes (mean 28 months). A decrease of >70% in the number of PVCs compared to the first Holter monitoring was observed in 34 (37%), while in 31 (33%), VAs did not show significant changes, and in 28 (30%), PVCs increased. During follow-up, no athlete presented with a major cardiac event.