The optimal duration of dual-antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation is an important, unanswered question. This study was designed to evaluate the association of varying durations of DAPT on clinical outcomes after DES implantation for the treatment of coronary artery disease. Using the National Heart, Lung, and Blood Institute Dynamic Registry, patients enrolled in the last 2 waves after index percutaneous coronary intervention with DES and who were event free at the time of landmark analysis were included. Landmark analysis was performed 12 and 24 months after percutaneous coronary intervention, and patients were stratified according to continued use of DAPT or not. Subjects were evaluated for rates of death, myocardial infarction, and stent thrombosis at 4 years from their index procedures. The numbers of evaluable patients were 2,157 and 1,918 for the 12- and 24-month landmarks, respectively. In both landmark analyses, there was a significantly lower 4-year rate of death or myocardial infarction in the group that continued DAPT compared to the group that did not (12 months: 10.5% vs 14.5%, p = 0.01; 24 months: 5.7% vs 8.6%, p = 0.02). Beneficial differences in the group that continued on DAPT were preserved after multivariate and propensity adjustment. There were no significant differences in definite stent thrombosis in either landmark analysis. In conclusion, at 12 and 24 months after DES implantation, continued use of DAPT was associated with lower 4-year risk for death and myocardial infarction.
The optimal duration of dual-antiplatelet therapy (DAPT) after the implantation of drug-eluting stents (DES) is unclear. On the basis of the initial randomized clinical trials of the first-generation DES, the United States Food and Drug Administration and American College of Cardiology and American Heart Association guidelines initially recommended DAPT for 6 months with paclitaxel-eluting (Taxus; Boston Scientific Corporation, Natick, Massachusetts) stents and 3 months with sirolimus-eluting (Cypher; Cordis Corporation, Miami Lakes, Florida) stents. Because of studies that suggested that patients who received 1 year of clopidogrel therapy after DES implantation had better survival at 2 years compared to those who received therapy for a shorter duration, current guidelines now recommend ≥1 year of DAPT for patients who receive DES if they are not at high risk for bleeding, and many have advocated for extending the duration of DAPT. Subsequently, several reports revealed conflicting information regarding the benefit of DAPT beyond 1 year after DES implantation. Other recent data from the Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) and the Efficacy of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting (EXCELLENT) trial suggest that shorter DAPT duration may be safe in selected patients. Because the duration of DAPT is still driven largely by individual physician and/or patient preference rather than evidence, we sought to determine the effect of varying durations of DAPT in unrestricted clinical practice on 4-year rates of death, myocardial infarction (MI), and stent thrombosis after DES implantation.
Methods
The National Heart, Lung, and Blood Institute Dynamic Registry is a multicenter North American registry that has been described in detail previously. Each center received institutional review board approval. Five recruitment waves of approximately 2,000 patients each have been enrolled and were followed. Only waves 4 and 5 are included in these analyses, because these were the waves in which DES were available. Patients in these 2 waves were recruited in 2004 and 2006, respectively.
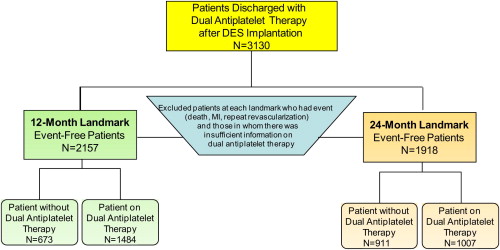
Patients who underwent successful implantation of ≥1 DES during their index percutaneous coronary intervention (PCI) and who were discharged on clopidogrel and aspirin were considered eligible for analysis ( Supplemental Figure 1 ). Patients who reported DAPT discontinuation and subsequently resumed it at a later time point during follow-up were excluded. Patients who received a combination of bare-metal stents and DES were included in the analysis given that the duration of DAPT would be driven by the placement of the DES.
Data on baseline demographic, clinical, angiographic, and procedural characteristics during the index PCI, as well as the occurrence of death, MI, and the need for repeat revascularization, were collected. At each follow-up time point, patients were asked to provide information regarding their medications. If the patients discontinued clopidogrel, they were asked the reason. With the use of the Social Security Administration’s Death Master File ( http://www.ntis.gov/products/ssa-dmf.aspx ), coordinators evaluated the vital status of patients who were lost to follow-up. If patients underwent subsequent repeat revascularization (either PCI or coronary artery bypass grafting), vessel-specific and lesion-specific data were collected whenever possible.
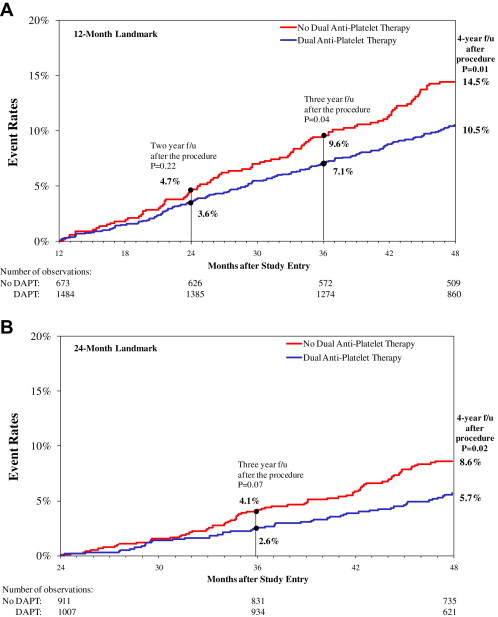
Landmark analysis allows the selection of patients who are “event free” at a specific time point after the index procedure and then following them forward. “Event free” is defined as the absence of death, nonfatal MI, or repeat revascularization. We analyzed the data using 12- and 24-month landmarks ( Figure 1 ) and evaluated outcomes at 4 years from the index procedure stratified by the use of DAPT. Individuals in whom there was insufficient information regarding their DAPT at either landmark time point were excluded from the analysis.
The primary end points were death and MI, and the secondary end point was repeat revascularization. At all landmark points, patients were stratified by whether they continued the use of DAPT or not, and descriptive statistics were summarized as means for continuous variables and percentages for categorical variables.
Differences between proportions were assessed using chi-square or Fisher’s exact tests, and continuous variables were compared using Wilcoxon’s nonparametric tests. Similar methods were used for lesion-level analyses. Unadjusted cumulative event rates for adverse outcomes at 3 years, for every landmark point, were calculated usng the Kaplan-Meier method, plotted, and compared using the log-rank statistic. Patients who did not experience the outcome of interest were censored at the last known date of contact or at 3 years if contact extended beyond 4 years.
The independent associations between DAPT use and 4-year death and death or MI were examined in 2 ways: (1) Cox proportional-hazards methods provided point estimates adjusted for important variables identified by forward stepwise selection (entry p-value criterion of ≤0.15, retain criteria of <0.05). Variables included in the model included demographics (age, renal disease, pulmonary disease, history of heart failure, and cancer), procedural variables (graft lesions, total occlusions, and calcified lesions), and discharge medications. (2) A propensity score approach was used to balance factors associated with the type of therapy. The estimated propensity score for continuation of DAPT was obtained from the fit of a logistic regression model for which demographic, angiographic, and procedural characteristics as well as discharge medications were considered. The proportionality assumption was assessed and met for all Cox proportional-hazards models. Hazard ratios with corresponding 95% confidence intervals are reported. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina), and a 2-sided p value ≤0.05 was considered for statistical significance.
Results
We identified 3,130 patients who received ≥1 DES and who were discharged on DAPT with aspirin and clopidogrel. For the 1-year landmark analysis ( Figure 1 ), 973 patients were not included in the analysis because they either experienced adverse events during the first year after their index PCI (death in 95, nonfatal MI in 120, and repeat revascularization in 242) or there was insufficient information regarding DAPT use. As a result, the 12-month landmark group included 2,157 patients with 1,484 (69%) who were continuing their DAPT use at 1 year and 673 (31%) who were not. For the 24-month landmark analysis ( Figure 1 ), 1,212 of the 3,130 DES-treated patients discharged with aspirin and clopidogrel were not included in the analysis because they either experienced events (death in 185, nonfatal MI in 154, and repeat revascularization in 327) during the 2 years after their index PCI or there was insufficient information regarding DAPT use. Accordingly, the 24-month landmark group included 1,918 patients, with 1,007 (53%) who were continuing their DAPT use at 2 years and 911 (47%) who had discontinued DAPT by 2 years. At the 12-month landmark, the subjects who continued DAPT included 24% of the wave 4 subjects and 44% of the wave 5 subjects. At the 24-month landmark, the subjects who continued DAPT included 17% of the wave 4 subjects and 35% of the wave 5 subjects.
Table 1 lists baseline characteristics for each landmark assignment. The mean age of the group that continued DAPT was slightly but significantly lower than that of the group that did not continue DAPT. For both landmark time points, patients receiving DAPT had a higher prevalence of diabetes and hyperlipidemia, although these differences were small. For the 2 analyses, patients receiving DAPT more often had histories of PCI.
| Variable | 12-Month Landmark | 24-Month Landmark | ||||
|---|---|---|---|---|---|---|
| Off DAPT (n = 673) | On DAPT (n = 1,484) | p Value | Off DAPT (n = 911) | On DAPT (n = 1,007) | p Value | |
| Baseline clinical and demographics | ||||||
| Mean age (yrs) | 65 | 63 | 0.003 | 64 | 63 | 0.02 |
| Women | 36% | 32.1% | 0.03 | 34% | 32% | 0.35 |
| White | 80% | 75% | 0.15 | 81% | 75.1% | 0.005 |
| Diabetes mellitus | 31% | 34.4% | 0.1 | 30% | 34% | 0.06 |
| Hypertension | 76% | 78.2% | 0.32 | 74% | 78% | 0.05 |
| Hypercholesterolemia | 77% | 79% | 0.36 | 76% | 79% | 0.08 |
| Smoking (current or former) | 64% | 65% | 0.74 | 65% | 63% | 0.69 |
| Renal disease | 9.9% | 7% | 0.04 | 8% | 7% | 0.34 |
| Cerebrovascular disease | 7% | 7% | 0.68 | 6% | 7% | 0.13 |
| Cancer | 8.2% | 6% | 0.11 | 8% | 6% | 0.03 |
| Peripheral vascular disease | 6% | 8.1% | 0.14 | 6% | 8% | 0.14 |
| Previous MI | 22% | 24% | 0.3 | 22% | 25% | 0.12 |
| Previous PCI | 25% | 35% | <0.001 | 26% | 35% | <0.001 |
| Mean left ventricular ejection fraction (%) | 54 | 52.9 | 0.64 | 54 | 54 | 0.5 |
| Lesion characteristics and procedural information | ||||||
| Procedure indication | 0.003 | 0.03 | ||||
| Acute MI | 26% | 28% | 27% | 28% | ||
| Unstable angina pectoris | 36% | 32% | 34% | 31% | ||
| Stable angina pectoris | 19% | 23% | 20% | 23% | ||
| Other | 19% | 17% | 19% | 18% | ||
| Number of vessels with >70% stenosis | 0.008 | 0.005 | ||||
| 1 | 55% | 47% | 54% | 47% | ||
| 2 | 28% | 31% | 29% | 32% | ||
| 3 | 16% | 19% | 16% | 19% | ||
| Circumstances of procedure | <0.001 | 0.008 | ||||
| Elective | 54% | 60% | 55% | 61% | ||
| Urgent | 37% | 29% | 35% | 28% | ||
| Emergent | 9% | 12% | 11% | 11% | ||
| Glycoprotein IIb/IIIa inhibitor use | 36% | 34% | 0.47 | 37% | 35% | 0.48 |
| PCI information | ||||||
| Mean number of DES stents | 1.5 | 1.6 | 0.01 | 1.5 | 1.6 | 0.02 |
| Patients treated with >1 DES | 35% | 40.8% | 0.01 | 36% | 41.3% | 0.02 |
| Patients treated with bare-metal stent and DES | 6% | 5% | 0.14 | 5.5% | 4.5% | 0.22 |
| PCI of proximal left anterior descending coronary artery | 16.6% | 15.8% | 0.37 | 16.9% | 15% | 0.22 |
| PCI of left main coronary artery | 1.5% | 3.3% | 0.02 | 2% | 3% | 0.15 |
| PCI of bifurcation lesions | 11% | 9% | 0.36 | 10% | 10% | 0.78 |
| Mean number of lesions attempted | 1.3 | 1.4 | 0.26 | 1.3 | 1.4 | 0.35 |
| Mean reference vessel size (mm) | 3 | 3 | 0.73 | 3 | 3 | 0.25 |
| Mean lesion length (mm) | 16 | 16 | 0.08 | 16 | 16.5 | 0.06 |
| American College of Cardiology/American Heart Association Classification | 0.79 | 0.24 | ||||
| Type A | 12% | 11% | 13% | 10.5% | ||
| Type B1 or B2 | 63% | 64% | 64% | 63% | ||
| Type C | 25% | 25.3% | 23% | 26% | ||
| Type of DES | ||||||
| Sirolimus | 58% | 60% | 0.34 | 59% | 60% | 0.45 |
| Paclitaxel | 35% | 35% | 0.93 | 35% | 35% | 0.85 |
| Discharge information | ||||||
| Bleeding before discharge | 5% | 4.2% | 0.76 | 4% | 4% | 0.98 |
| Medications on discharge | ||||||
| Statins | 85% | 85.9% | 0.46 | 85.8% | 86% | 0.98 |
| β blockers | 81% | 82.3% | 0.57 | 80% | 83% | 0.08 |
| Angiotensin-converting enzyme inhibitors | 52% | 52.4% | 0.86 | 51% | 52.3% | 0.5 |
| Warfarin | 9% | 5% | <0.001 | 8% | 4% | <0.001 |
For the group that did not continue DAPT, reasons for cessation of clopidogrel were available only for patients from wave 5 ( Supplemental Tables 1a and 1b ). In this wave, discontinuation of clopidogrel was physician mediated in approximately 90% of patients and due to noncompliance in only about 5% of patients.
With respect to lesion and procedural characteristics ( Table 1 ), at both landmarks, only minor differences were noted in procedural indications, while there was a greater prevalence of multivessel coronary artery disease and implantation of >1 DES among those patients who continued DAPT. There were no significant differences at either landmark time point with respect to the use of glycoprotein IIb/IIIa inhibitors, the number of lesions attempted, reference vessel size, lesion length, or American College of Cardiology and American Heart Association lesion type classification. There were no differences in the type of DES used. Procedural success was high in the 2 groups, and rates of periprocedural bleeding were similar. There were no important differences in medications used on discharge, except that more patients who discontinued DAPT earlier had been discharged on warfarin.
For the entire cohort of 3,130 patients who were treated with DES and discharged on DAPT, the 4-year cumulative rates of death, MI, repeat revascularization, and stent thrombosis were 9.1%, 7.5%, 18.8%, and 1.4%, respectively.
Figure 2 shows the 4-year Kaplan-Meier curves for death and MI for patients in the 12-month landmark analysis. Those who continued DAPT had a significantly lower risk for 4-year death and MI compared to those who did not (10.5% vs 14.5%, p = 0.01). One-year after the landmark, the differences between the 2 groups were small and nonsignificant, but the benefit of continuation of DAPT was most appreciated with longer term follow-up. Overall, for the group that did not continue DAPT, the annual rate of death or MI was approximately 4.7% per year, while the group that continued on DAPT had an annual event rate of 3.6% per year. The cumulative event rates for the 12-month landmark groups are listed in Table 2 . Risk for death was lower in the group that continued DAPT, but the rate of repeat revascularization in the 2 years after the landmark was higher in these patients. No significant differences were noted in rates of definite stent thrombosis between the group that continued DAPT and the group that did not.