CHAPTER 94 Nonatherosclerotic Coronary Heart Disease
Although myocardial ischemic syndromes (angina pectoris, myocardial infarction, and sudden cardiac death) are almost always caused by coronary atherosclerosis, they occasionally result from one of a variety of nonatherosclerotic coronary artery diseases.1–3 Less than 5% of all patients presenting with acute myocardial infarction do not have atherosclerotic disease as a cause of luminal narrowing, as demonstrated at autopsy; however, this may be an overestimate when spontaneous reperfusion and lysis of acute coronary thrombosis are also considered.4 These relatively uncommon diseases pose several problems to the clinician:
CORONARY ARTERY ANOMALIES
Coronary artery anomalies (i.e., variations in the origin, course, or distribution of the coronary arteries) are present in 1% to 2% of the population.5 These anomalies may make angiographic visualization of the coronary circulation more difficult and selective engagement of ostia more challenging, and they may increase the risk of coronary artery trauma during cardiac surgery.2 In addition, certain types of coronary anomalies may cause myocardial ischemia,6 although most do so in a small fraction of the patients in whom they are present. Evaluation of each patient with a coronary anomaly should therefore include anatomic classification, recognition of the particular anomaly and its potential to result in myocardial ischemia, and documentation that ischemia is present, using functional and perfusion imaging techniques. In the symptomatic patient with a coronary anomaly in whom ischemia has been documented by exercise testing, nuclear myocardial perfusion scanning, stress echocardiography, or transmyocardial metabolic testing, effective corrective treatment is generally possible. Magnetic resonance imaging and multidetector computed tomographic angiography are emerging as standard modalities for the diagnosis of these anomalies, for their classification, and potentially for guiding therapy.
Anomalous Origin from the Aorta
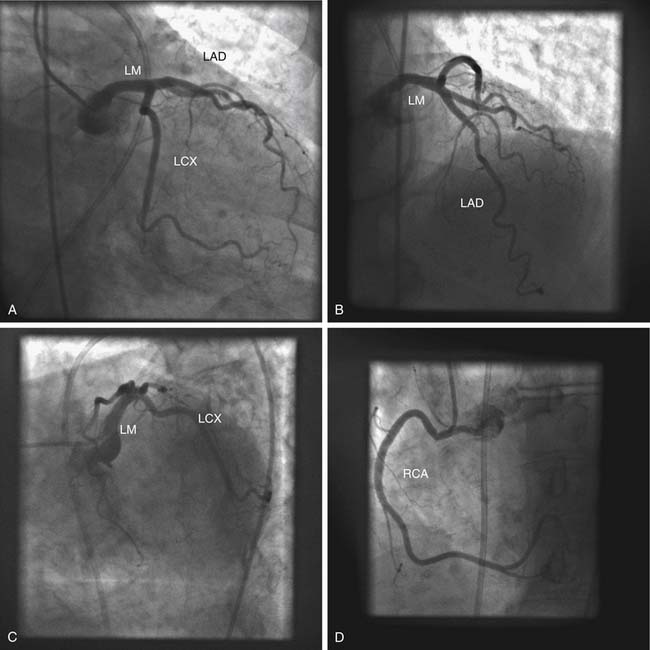
In normal coronary circulation, the right coronary artery originates from a single ostium in the right sinus of Valsalva, and the left coronary artery originates from a single ostium in the left sinus of Valsalva (Fig. 94-1). Abnormally high or low locations of the coronary ostia and the presence in the appropriate sinus of Valsalva of separate ostia for the left anterior descending and circumflex coronary artery branches, or for the right coronary artery and its conus branch, are common minor variations that do not result in myocardial ischemia. Other anomalous patterns of coronary artery origin from the aorta are potential causes of myocardial ischemia, even in the absence of atherosclerosis.
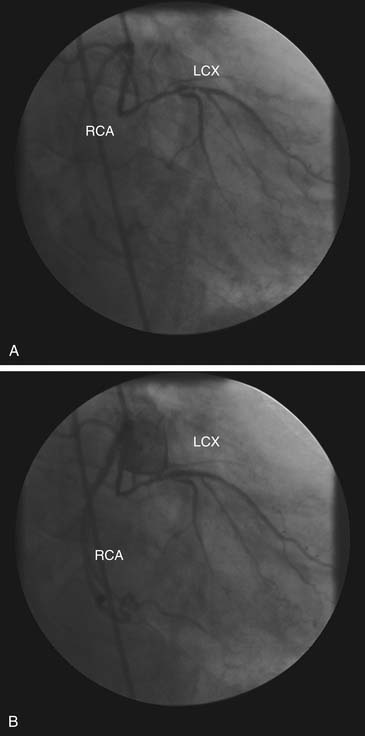
Origin from the Contralateral Sinus of Valsalva
When one of the coronary arteries originates from the contralateral sinus of Valsalva, this anomalous vessel must traverse the base of the heart to reach its territory of distribution by passing anterior to, posterior to, or between the aorta and the pulmonary artery (Fig. 94-2).7,8 Acute angulation at the origin of the artery from the aorta may result in anatomic or functional constriction of the proximal portion of the anomalous coronary artery. Increased cardiac output (e.g., as a result of exercise) causes aortic dilation and narrowing at the area of angulation at the abnormal coronary ostium. Anomalous vessels passing between the aorta and the pulmonary artery seem to carry an additional risk of ischemia, possibly as a result of being compressed between the great vessels; however, this is unlikely at normal pulmonary artery pressures.
Abnormal mechanical stresses or flow patterns may enhance the development of coronary atherosclerosis in the anomalous segment. Origin of the left coronary artery from the right sinus of Valsalva with passage of the proximal left coronary artery between the aorta and pulmonary artery is associated with an increased incidence of exercise-related sudden cardiac death in young patients. In an autopsy study of 33 patients with this anomaly, sudden death occurred in nine patients (27%), generally without prior warning symptoms. This is, however, a selected group of patients, because diagnosis at this age requires the presence of symptoms and exercise test abnormalities that lead to the diagnostic angiography.1 In patients without coronary atherosclerosis, passage of the anomalous left coronary artery either anterior or posterior to both great vessels has been associated with pacing-induced myocardial lactate production and angina pectoris.7,9 Thus, some recommend prophylactic coronary artery bypass surgery when this anomaly is detected in young patients.6,8
In a study of nine patients who underwent a modified unroofing procedure to move the coronary artery orifice to the appropriate sinus, all nine were free of symptoms and demonstrated no abnormalities on echocardiography suggestive of ischemia at 2-year follow-up.10 Angina pectoris has also been reported in patients in whom the right coronary artery originates from the left sinus of Valsalva, but confirmation of myocardial ischemia has been less complete in these patients than in patients with anomalous origin of the left coronary artery.7 The most common pattern of anomalous aortic origin—origin of the circumflex coronary artery from the right sinus of Valsalva or proximal right coronary artery—does not seem to impose independent ischemic risk.7 Although stenting has been used in small series and case reports, its effectiveness has not been demonstrated, and it should be used only with careful follow-up.
Single Coronary Artery
Derivation of the entire coronary circulation from a single ostium is a rare coronary anomaly. Approximately 40% of patients with this anomaly have an associated congenital cardiac defect (e.g., tetralogy of Fallot, transposition of the great vessels, improper division of the truncus arteriosus).11 There is no clear sex predominance for this condition, and the frequency of occurrence of single left and single right coronary arteries is approximately equal.12 As is also true in the case of anomalous origin from the contralateral sinus of Valsalva, one or more components of the coronary circulation system must cross the base of the heart to reach its territory of distribution by passing anterior to, posterior to, or between the great vessels. These transposed vessels may therefore be exposed to the risks of angulation, compression, and accelerated atherosclerosis. Because the entire myocardium is supplied by way of the single coronary artery, proximal coronary atherosclerosis poses the risk of global myocardial ischemia. Clinical manifestations of the single coronary artery anomaly depend in part on the associated cardiac defects and atherosclerosis, but up to 15% of patients with only this anomaly develop severe cardiac complications by age 40.11 Angina pectoris and myocardial lactate production have been demonstrated in patients with a single coronary artery in the absence of coronary atherosclerosis or vessel passage between the aorta and the pulmonary artery.12
Atresia of the Coronary Ostium
Atresia or severe stenosis of one of the coronary ostia (often associated with hypoplasia of the proximal coronary artery) is a rare congenital coronary anomaly, with only seven reported cases.6,13 The absence of a second coronary ostium may lead to the incorrect diagnosis of a single coronary artery. Because the involved vessel depends on collateral flow from the contralateral coronary artery, myocardial ischemia or infarction may develop during infancy. In this sense, patients with ostial atresia bear an angiographic and clinical resemblance to patients with an anomalous origin of a coronary artery from the pulmonary artery (see later). Successful coronary artery bypass grafting has been reported in a 10-year-old boy with ostial atresia.13
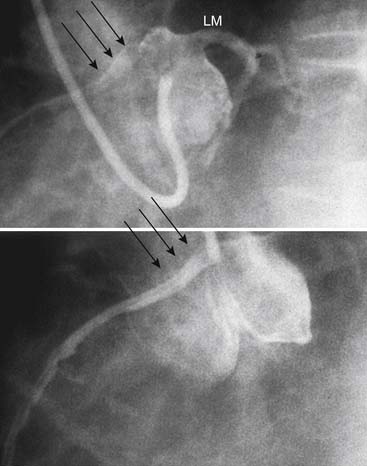
High Takeoff Coronary Ostia
The location of the coronary arteries in the sinuses of Valsalva allows maximal diastolic filling. In some cases, the ostia have been reported to be located well above the sinuses (Fig. 94-3) and associated with decreased coronary perfusion and myocardial ischemia. This theory has been challenged by the normal flow pattern seen in these arteries as well as in vein grafts and arterial conduits located well above the sinuses.14
Anomalous Origin from the Pulmonary Artery
Origin of a coronary artery from the pulmonary artery rather than from the aorta is a relatively uncommon but severe coronary anomaly that occurs in approximately 1 out of every 300,000 live births (0.25% to 0.5%). However, it is one of the most common causes of myocardial ischemia and infarction in children.15 In more than 90% of cases, it is the left coronary artery that originates from the pulmonary artery, generally from the left posterior pulmonary sinus.6 Origin of the right coronary artery, an accessory coronary artery, and both coronary arteries from the pulmonary artery have been described; the last of these is invariably fatal during the neonatal period.6,16
Edwards17 first explained the pathophysiology of the hemodynamics that occur with the anomalous origin of the left coronary artery from the pulmonary artery. As the pulmonary artery pressure falls during the first weeks of life, perfusion of the anomalous coronary artery from the pulmonary artery decreases. Unless adequate collateral flow develops from the contralateral coronary artery, the territory of the anomalous vessel becomes ischemic. Angina pectoris or congestive heart failure with mitral regurgitation may then develop, and this is often accompanied by the electrocardiographic manifestation of myocardial ischemia or infarction.
The clinical picture, called the infantile syndrome, develops in approximately 80% of affected patients, usually within the first 4 months of life.6,15 The typical presentation in infants includes failure to thrive, profuse sweating, dyspnea, pallor, and atypical chest pain on eating or crying.18 In the absence of surgical correction, this syndrome has an 85% first-year mortality, although the mortality rate is somewhat lower with anomalous origin of the right coronary artery.16 Those who do not develop the infantile syndrome may present during childhood or adult life with one of the following: asymptomatic murmur, mitral regurgitation, angina pectoris, or sudden death. In these patients, there is a greater than 80% incidence of sudden death at a mean age of 35 years.15 Patients who survive infancy tend to have extensive intercoronary collateral flow, with dilation of both the normal and the anomalous vessels. This collateral flow reverses the direction of blood flow in the anomalous coronary artery, which constitutes a left-to-right shunt into the pulmonary artery. Despite extensive collateralization, electrocardiographic evidence of ischemia and pathologic evidence of subendocardial fibrosis usually persist. Diagnosis can generally be made on the basis of electrocardiographic and echocardiographic findings. When there is uncertainty, angiography is warranted. Medical management is associated with a high mortality rate, and the current standard of care calls for immediate surgical repair at the time of diagnosis.18
Surgical correction seeks to eliminate the left-to-right shunt and to establish an independent arterial blood supply to the anomalous vessel. A variety of techniques have been attempted since the earliest operative attempts by William J. Potts. Ligation of the anomalous vessel at its origin in combination with saphenous vein aortocoronary bypass grafting has been performed, but it is technically difficult in children who are less than 2 years old, and it is associated with a high rate of graft failure.19 Currently, the most popular method reestablishes a two-coronary system via reimplantation of the anomalous vessel into the aortic root. Improvisation is sometimes necessary because of anatomic variations and insufficient coronary artery length. Overall, across a variety of series, the mortality rate of this procedure ranges from 0% to 16%.18 Michielon and colleagues20 followed 30 patients for an average of 15 years after surgery. They showed that the coronary transfer technique demonstrated the best freedom from long-term reoperation (92.3% ± 7.4%) as compared with subclavian interposition and intrapulmonary tunnel. Additionally, when surgery was performed before the patient was 6 months old, significantly improved left ventricular and mitral valve function was achieved, despite worse preoperative left ventricular function than that seen in older patients. Continued improvement in left ventricle function is seen up to 1 year after surgery. Because of the high morbidity and mortality rates of this coronary artery anomaly, aggressive early operative management that reestablishes a two-coronary system is warranted to achieve the best functional recovery. Most of these patients recover normal mitral valve function after reperfusion alone. Despite the presence of preoperative mitral insufficiency, mitral valve repair at the time of initial repair is generally not recommended.
Coronary Artery Fistula
Direct precapillary anastomosis between a major coronary artery and a cardiac chamber or major vessel (e.g., superior vena cava, coronary sinus, pulmonary artery) is the most common hemodynamically significant coronary artery anomaly.6 These communications are often referred to as coronary–cameral fistulas, and they occur in approximately 1 out of every 50,000 patients with congenital heart disease. Fistulas from the right coronary artery are slightly more common than those from the left coronary artery, and bilateral fistulas are present in 4% to 5% of cases.21 More than 90% of the fistulas drain into the venous circulation (right ventricle, 41%; right atrium, 26%; pulmonary artery, 17%; coronary sinus, 7%; superior vena cava, 1%), and the remaining fistulas drain into the arterial circulation (left atrium, 5%; left ventricle, 3%).6 Multiple patterns of anastomosis between the involved coronary artery and the recipient cardiac structure are possible.
In addition to congenital fistulas, coronary artery fistulas can also be acquired as a result of trauma, angioplasty, transcutaneous catheter techniques for myocardial biopsies, and heart operations.22 The involved coronary artery is usually markedly dilated proximal to the fistula, if hemodynamically significant, and flow through the fistula may be several times that delivered to the myocardium. When the fistula drains into the venous circulation, a significant left-to-right shunt may be present.23 Runoff through a fistula may lower intracoronary diastolic pressure and produce myocardial ischemia in some patients by the coronary steal phenomenon.6 However, the proximal coronary tree dilates and the corresponding blood flow usually increases to accommodate total flow, so steal occurs mainly with development of atherosclerosis or with reduced cardiac output.
Because the great majority of patients with coronary fistulas are asymptomatic, the decisions surrounding surgical correction are complex. Even in asymptomatic patients, clinical, electrocardiographic, and roentgenographic abnormalities are often present. Ischemia has been documented in some patients with coronary fistulas and no atherosclerosis,6 and some evidence suggests that with a significant fistula, patients do become symptomatic with advancing age.23 In addition to angina and myocardial infarction, congestive heart failure, bacterial endocarditis, and fistula rupture have been described. Antibiotic prophylaxis against bacterial endocarditis is no longer recommended without any preceding endocarditis or complex congenital heart disease.
Because spontaneous fistula closure is rare, and because the risk of surgical closure of the fistula is significantly lower in patients who are less than 20 years old, some have suggested elective fistula ligation in young patients, including those patients who are asymptomatic.23 Surgical closure is a safe and effective treatment. In a retrospective review over 28 years, Mavroudis and colleagues22 demonstrated a 100% survival rate and a 100% closure rate after surgical ligation of the fistula. However, as this seems a bit too invasive for a condition that may not develop, catheter-based techniques have been developed. These include coils, detachable balloons, vascular plugs, and double-umbrella devices.24,25 Criteria for transcatheter treatment include the absence of multiple fistulae, a single narrow drainage site, the absence of large branch vessels, and safe accessibility to the coronary artery that supplies the fistula.22 Complications (less frequent with increasing experience) seen with transcatheter techniques include the migration of coils, transient arrhythmias, fistula dissection, and residual shunts.22,24,25 Residual shunts pose a problem given the risk of bacterial endocarditis and the presence of a foreign body; repeat catheterization and occlusion are therefore recommended.24 The exact role of surgical versus transcatheter therapy for coronary artery fistulas is still being debated. As transcatheter and imaging techniques improve, they will play a greater role in treatment strategies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree