Non-ST Elevation Myocardial Infarction and Unstable Angina
Jacqueline E. Tamis-Holland
Amy Chorzempa
EPIDEMIOLOGY
Unstable angina (UA) and non-ST-segment elevation myocardial infarction (NSTEMI) are part of the clinical spectrum known as acute coronary syndromes (ACS). They are often grouped together as one entity owing to their similar presenting characteristics and pathophysiology and are collectively referred to as the non-ST-segment elevation ACS (NSTE-ACS). NSTE-ACS is a highly prevalent and serious manifestation of coronary heart disease. There are approximately 1.36 million hospitalizations in the United States alone (765,000 males and 600,000 females) for all ACS (including ST-elevation MI [STEMI] and NSTE-ACS).1 The estimated proportion of all ACS patients who present with NSTE-ACS varies with different registries; in the second Euro-Heart Survey, 53% of all cases of ACS were NSTE-ACS, whereas 68% of ACS cases reported by the AHA Get with the Guidelines Project, and 62% of cases reported in the study of the Global Registry of Acute Coronary Events (GRACE) were NSTE-ACS.2
PATHOPHYSIOLOGY
NSTE-ACS often occurs when there is an imbalance of myocardial oxygen supply and demand resulting in myocardial ischemia. By definition, UA is angina that has changed in quality. This includes new onset angina, angina that occurs with little or no activity, or prolonged episodes of angina. When myocardial ischemia is very prolonged, myocardial necrosis ensues. This is referred to as NSTEMI. Most cases of UA and NSTEMI are the result of severe atherosclerotic plaque causing impingement of the arterial lumen and partially impairing myocardial blood flow. Typically, atherosclerotic plaques comprise cellular debris, cholesterol, and a fibrous cap. In some cases, platelets accumulate on the surface of a ruptured plaque resulting in transient clot formation with occlusion of the lumen or distal micro-emboli and myocardial necrosis. NSTE-ACS can sometimes be seen in situations where there is less severe epicardial coronary artery obstruction. This includes (1) smooth muscle cell spasm with transient arterial occlusion (vasospastic angina); (2) moderate or severe obstruction accompanied by a severe imbalance in myocardial blood supply and demand (as a result of severe anemia, severe hypotension, or hypertensive crisis); (3) inflammation of the blood vessel walls resulting in thickening of the walls and luminal narrowing (vasculitis); or (4) conditions resulting in paradoxical coronary emboli such as atrial fibrillation, atrial myxoma, left ventricular (LV) clot, or vegetations of the heart valves.
DIAGNOSIS
HISTORY AND PHYSICAL EXAM
It is important that physicians maintain a high index of suspicion for all suspected cases of NSTE-ACS, so that rapid diagnosis can be made, allowing for early institution of medical therapies. Obtaining a detailed clinical history should be the first step in making the diagnosis. Myocardial ischemia typically produces symptoms that are commonly described as chest “squeezing,” “heaviness,” or “pressure,” although it is not uncommon for patients to complain of a “stabbing,” “sharp,” “aching” or “burning” chest pain. Symptoms may radiate to the jaw, left arm/shoulder, or the back, and may be accompanied by shortness of breath, nausea, or diaphoresis. A history of progressive pain that is aggravated by physical activity or relieved with rest and culminates in symptoms with minimal activity or resting pain is very suggestive of UA, although many patients may present for the first time with rest symptoms. Not all patients have these classic symptoms, and NSTE-ACS should still be considered in patients who present only with shortness of breath, epigastric pain, or back pain. Up to 20% of patients with ACS do not have chest pain on hospital presentation. Older patients, female patients, and patients with diabetes mellitus are more likely to have no chest pain.3 The remainder of the clinical history should be focused on related cardiac risk factors, and prior cardiac conditions including prior MI, percutaneous coronary interventions or coronary artery bypass (CABG) surgery as this will help to define a patients’ cardiac risk. (See “Risk Stratification.”)
The physical exam does not usually help in the diagnosis of NSTE-ACS as most patients have an unremarkable exam. However, the abnormalities including tachycardia, hypotension, heart murmurs, respiratory crackles, or poor distal pulses may indicate that the patient is at higher risk and therefore warrant therapies that are more aggressive.
ELECTROCARDIOGRAM
The ACC/AHA guidelines for the management of patients with UA/NSTEMI state that a 12-lead ECG should be performed as soon as possible after arrival in the emergency room (ER) and
ideally within 10 minutes of presentation for all patients with suspected ACS.4 This will allow for the immediate differentiation of patients with NSTE-ACS from those who have STEMI, as initial therapies will vary depending on the presenting type of ACS. ECG changes that are considered suspicious for NSTEACS include ST segment depressions of ≥0.5 to 1 mm or T-wave inversions of ≥2 mm in two or more contiguous leads. It is not uncommon however for a patient to have a normal ECG.
ideally within 10 minutes of presentation for all patients with suspected ACS.4 This will allow for the immediate differentiation of patients with NSTE-ACS from those who have STEMI, as initial therapies will vary depending on the presenting type of ACS. ECG changes that are considered suspicious for NSTEACS include ST segment depressions of ≥0.5 to 1 mm or T-wave inversions of ≥2 mm in two or more contiguous leads. It is not uncommon however for a patient to have a normal ECG.
CARDIAC BIOMARKERS
The cardiac biomarkers are intracellular macromolecules that are released into the blood stream when myocardial necrosis and cell death occurs. The cardiac biomarkers, most commonly used today, include the cardiac troponins and creatinine phosphokinase (CPK-MB). There are three components of the troponin gene, Troponin T, Troponin I, and Troponin C. Troponin C can be found in both cardiac and skeletal muscles, whereas Troponin T or I are derived from “heart specific genes” and hence are collectively referred to as the cardiac troponins. The cardiac troponin assays are extremely sensitive and specific and are the test of choice when assessing for myocardial necrosis. Cardiac troponin elevations directly correlate with the degree of myocardial injury. There is an independent increased risk for death among the troponin positive patients when compared with those patients with negative troponins. Furthermore, there is an incremental relationship between the risk of death and the cardiac troponin level.5 CPK-MB is a cytosolic carrier protein for high-energy phosphates. It is less sensitive and less specific for myocardial necrosis than the cardiac troponins; low levels of CPK-MB are sometimes found in the blood of healthy athletes or in association with skeletal muscle damage. CPK-MB should be used in cases when troponin assays are not available.
The cardiac biomarkers can be detected in the blood at anytime between 2 and 12 hours following myocardial necrosis. The cardiac troponin levels generally return to baseline at 4 to 14 days after the index event, whereas the CPK-MB levels will normalize much earlier (within 2 to 3 days).6 Therefore, the CPK-MB is useful when evaluating patients with suspected reinfarction. Cardiac biomarkers should be obtained in all patients with suspected ACS. This allows the physician to classify patients further into UA and NSTEMI and provides the physician with an important tool used for risk stratification. It is recommended that a patient have an initial blood test on presentation and that this blood test be repeated 6 to 9 hours after admission and then again 12 to 24 hours.
RISK STRATIFICATION
There are two main questions one should ask when examining a patient with suspected ACS: (1) Are the symptoms the patient complains of indicative of cardiac ischemia? (2) If this is an ischemic event, what is the likelihood that this patient will suffer an adverse outcome from this event? The initial assessment will help to answer the former question and provide the clinician with a potential working diagnosis including noncardiac chest pain, and rule out ACS, UA, or NSTEMI. Assuming a working diagnosis of NSTE-ACS based on the initial assessment, the next step is to perform a detailed and accurate risk assessment. Risk stratification provides the clinician with important information about a patient’s prognosis and guides the use of subsequent medical and invasive therapies. For example, high-risk patients are usually treated with IV glycoprotein IIb/IIIa (GP IIb/IIIa) receptor antagonists and early catheterization. (See “Medical Treatment-Antiplatelet Therapy and Initial Conservative versus Initial Invasive Approach.”)
The information used to estimate a patient’s risk is derived from the patient’s clinical history, physical exam, ECG findings, and cardiac biomarkers. In general, a patient can be stratified into three categories: low, intermediate, or high-risk groups. Low-risk patients include younger patients with a normal ECG who do not have any prolonged or recurrent symptoms, have no physical signs of CHF or shock, and have normal cardiac biomarkers. High-risk patients generally have evidence of extensive ischemia or infarction, with pathologic ST and T wave changes on ECG, or moderately to severely elevated cardiac biomarkers, or congestive heart failure/shock. Patients at intermediate risk are those who fall in between these categories.
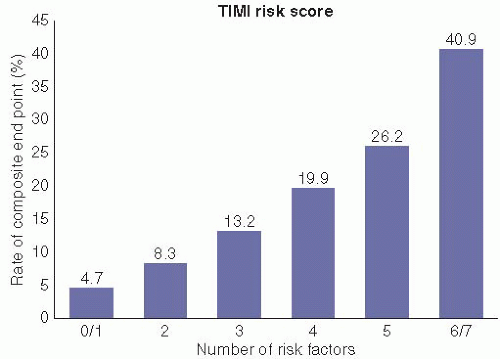
Cardiac risk scores are simple tools that allow the clinician to estimate qualitatively a patient’s risk based on the interplay of multiple clinical variables. There are various risk scores that have been derived and shown to correlate with clinical outcomes.7,8,9 and 10 The Thrombolysis In Myocardial Infarction (TIMI) risk score7 is the most commonly used tool for risk assessment and has been shown to have superior prognostic accuracy to that obtained using a physician’s “in-formal” assessment.11 This is a simple 7-point system that provides an estimate of a patient’s risk of death, recurrent MI, or severe refractory ischemia requiring urgent revascularization at 2 weeks. The variables that are used in the score include (1) age >65 years, (2) prior known coronary stenosis of >50%, (3) the presence of at least three coronary artery disease risk factors, (4) elevated cardiac enzymes, (5) ST-segment deviation on ECG, (6) recurrent angina prior to presentation, and (7) aspirin use within the last week. One point is allocated for each of the seven variables used in the score, resulting in a total possible score of 7. Event rates increase significantly with increasing risk score (Figure 3.1). As an example, a 60-year-old otherwise healthy
male patient who has no previous medical history and is not taking any medications presents with onset of chest pain lasting for 20 minutes, without recurrence. His ECG and cardiac troponins are all normal. His TIMI risk score is zero, and he has a low likelihood for a cardiac event. However, a 78-year-old male with prior CABG surgery on aspirin presents with recurrent resting chest pain, ST-segment depression on ECG, and elevated cardiac troponins. He has a score of 6. Among patients like him, 40% will suffer a cardiac event in the ensuing 2 weeks.
male patient who has no previous medical history and is not taking any medications presents with onset of chest pain lasting for 20 minutes, without recurrence. His ECG and cardiac troponins are all normal. His TIMI risk score is zero, and he has a low likelihood for a cardiac event. However, a 78-year-old male with prior CABG surgery on aspirin presents with recurrent resting chest pain, ST-segment depression on ECG, and elevated cardiac troponins. He has a score of 6. Among patients like him, 40% will suffer a cardiac event in the ensuing 2 weeks.
The GRACE risk score8 was designed to assess the likelihood of in-hospital death among patients with ACS including STEMI, NSTEMI, or UA using variables from an unselected population of patients enrolled in an international clinical registry. Although the TIMI risk score assigns an equal value to each risk, the GRACE risk score assigns values to each variable depending on the strength of the risk. In addition, because the GRACE score was derived from an unselected population of patients, it incorporates a larger range of clinical variables than the TIMI risk score including congestive heart failure, chronic kidney disease, and resuscitated cardiac arrest. In this score eight factors were identified as important predictors of risk (http://www.outcomes-umassmed.org/grace/). The GRACE risk score has been shown to have superior ability to differentiate risk groups and subsequent outcomes when compared with other risk scores.12,13
MEDICAL TREATMENT
The goal of medical therapy is to stabilize the patient, by relieving chest pain/symptoms (anti-ischemic agents) and protecting the patient from subsequent events including recurrent ischemia, MI, or death (antiplatelet/anticoagulants and statins).
ANTI-ISCHEMIC AGENTS
Oxygen. Oxygen use may cause comfort and provide relief from breathlessness that patients with myocardial ischemia sometimes feel. It should be used routinely in patients with NSTEACS who present with hypoxemia or respiratory distress.4 Oxygen should be administered with caution to patients with concomitant chronic obstructive pulmonary disease (COPD).
Nitroglycerin. Nitroglycerin causes nitric-oxide-mediated vasorelaxation, with effects on both the coronary and peripheral vascular beds. By dilating the venous circulation, it results in a reduction in preload, which decreases wall stretch, and results in a decrease in myocardial oxygen demand and improvement in ischemic symptoms. Nitroglycerin causes a more modest reduction in afterload, further reducing myocardial oxygen demand. Nitroglycerin has direct effects on the epicardial coronary arteries, with dilatation of normal or diseased vessels. Because of the effects on the venous and arterial circulation, nitroglycerin is used to treat concomitant hypertension or congestive heart failure too. There is no role for the routine initiation of nitroglycerin in the absence of ischemic symptoms, congestive heart failure, or hypertension.14
Nitroglycerin should be administered sublingually for relief of ischemic chest pain at a dose of 0.4 mg every 5 minutes. The patient’s blood pressure should be monitored carefully between each dose. After initial treatment with three sublingual tablets, consideration should be given to the initiation of IV nitroglycerin if chest pain persists. IV nitroglycerin should be administered at a starting dose of 10 μg per minute and increased by 10 to 20 μg per minute every 3 to 5 minutes as needed to control symptoms while maintaining adequate blood pressure. Once stabilized, a transition to oral long-acting formulations or trans-dermal dosing of nitroglycerin may be considered when clinically indicated for the treatment of intermittent chest pain, or in cases where optimization of medical therapy is planned.
Nitroglycerin should not be used in patients with hypotension or in patients with suspected right ventricular (RV) infarction/ischemia or volume depletion as the negative effect on preload may exacerbate hypotension. In addition, because phosphodiesterase inhibitors cause smooth muscle cell relaxation, they may potentiate the vasodilating effects of nitroglycerin. Therefore, nitroglycerin should also be avoided in patients who have recently taken an oral phosphodiesterase inhibitor.
Morphine. There is invariably some degree of discomfort as well as anxiety and apprehension in most of the patients presenting with NSTE-ACS. This often leads to an increase in sympathetic tone that can exacerbate the supply demand imbalance already seen in the ischemic myocardium. Morphine sulfate is the preferred agent for the relief of pain and anxiety related to ischemic chest discomfort. It can be administered intravenously at starting doses of 1 to 2 mg and repeated as needed to achieve the desired effect of pain relief. Morphine causes veno-dilation with a resultant decrease in preload and therefore is of special benefit in those patients with accompanying acute pulmonary edema or congestive heart failure. Morphine can lower blood pressure, and on occasion cause bradycardia (through increased vagal tone) or respiratory depression. Therefore, a patient’s vital status should be carefully monitored for signs or symptoms of hypotension, bradycardia, or respiratory depression. Naloxone can be administered intravenously in doses of 0.1 to 0.2 mg every 15 minutes when indicated to reverse the narcotic effects of morphine. Of note, in a large observational registry of 443 hospitals in the United States enrolling 53,039 patients with NSTE-ACS,15 the use of IV morphine sulfate was associated with a higher adjusted likelihood of death (propensity adjusted OR: 1.41, 95% CI 1.26, 1.56). Although not randomized, this information raises some concern regarding the use of morphine in patients with ACS.
β-Adrenergic blocking agents. β-Adrenergic blocking agents work by competitively blocking the effects of catecholamines on the β-receptors of cell membranes. Blockade of the B-1 receptors on cardiac myocytes produces slowing of the heart rate and decreased myocardial contractility. This results in decreased myocardial oxygen demand, which favorably shifts the supply demand imbalance toward decreased demand. β-Blockers are therefore indicated for the initial treatment of patients with presumed ACS. Oral β-adrenergic blocking agents should be initiated within the first 24 hours of presentation in the absence of a contraindication to use including hypotension, bradycardia (or high-grade atrioventricular block), congestive heart failure, or severe symptomatic reactive airway disease.
The utility of early intravenous β-blockers has been challenged recently by results of the ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) study. In this large randomized trial16 45,852 patients with acute MI (93% STEMI and 7% NSTEMI) were randomized to treatment with 15 mg of intravenous metoprolol given over 15 minutes followed by a dose of 200 mg of extended release oral metoprolol daily or placebo. The use of early intravenous β-blockers had a neutral
effect on the combined endpoint of mortality, recurrent infarction, or cardiac arrest. There was a lower incidence of recurrent infarction in the treated group (2.0% vs. 2.5% P = .0002) that was balanced by a significantly higher rate of cardiogenic shock in this same group (5.0% vs. 3.9% P < .00001). Therefore, intravenous β-blockers should be reserved for those patients with refractory hypertension who do not appear to be at increased risk for developing cardiogenic shock.
effect on the combined endpoint of mortality, recurrent infarction, or cardiac arrest. There was a lower incidence of recurrent infarction in the treated group (2.0% vs. 2.5% P = .0002) that was balanced by a significantly higher rate of cardiogenic shock in this same group (5.0% vs. 3.9% P < .00001). Therefore, intravenous β-blockers should be reserved for those patients with refractory hypertension who do not appear to be at increased risk for developing cardiogenic shock.
There are various β-blocker regimens available to physicians for use, with only slight differences in their physical properties. The decision to use one β-blocking agent over another will be largely based on individual patient characteristics and physician/hospital preference: For example, when instituting a β-blocking agent, one may wish to use a short-acting regimen while titrating the initial dose. In addition, if a patient has a history of reactive airway disease, one might choose a predominantly β-1 selective β-blocker (metoprolol and atenolol) to minimize the β-2 effects of these agents that might potentiate bronchoconstriction. Finally, if a patient has a history of congestive heart failure or LV dysfunction one might consider the use of carvedilol or long-acting metoprolol as they have been proven beneficial for use in patients with compensated heart failure.17,18 and 19
Calcium channel blockers. Calcium channel blockers prevent the influx of calcium across the smooth muscle cell membrane thereby causing relaxation of the smooth muscle cells. There are two general classes of calcium channel blockers, the dihydropyridine calcium antagonists (nifedipine, felodipine, and amlodipine) and the non-dihydropyridine calcium antagonists (verapamil and cardizem). The dihydropyridine calcium antagonists predominantly exert their effects through relaxation of the peripheral vascular smooth muscle cells, whereas the nondihydropyridines predominantly work by reducing myocardial contractility and AV nodal conduction. Both classes of agents have some effects on coronary vasodilatation. Small randomized controlled studies performed in the 1980s demonstrated that calcium channel blockers decrease ischemic symptoms and improve outcome in patients with UA or NSTEMI.20,21 However, the short-acting dihydropyridine calcium channel blockers can cause a reflex tachycardia, thereby worsening ischemia. In the absence of concomitant β-blocker use the dihydropyridines have been shown to have a negative impact on outcome.22 However, both verapamil and diltiazem have the potential to worsen congestive heart failure in patients with significant LV dysfunction. Therefore, calcium channel blockers are generally not considered first line therapy for the treatment of NSTE-ACS. However, if a patient has significant reactive airway disease preventing the administration of a β-blocker, a non-dihydropyridine may be considered as an alternative antiischemic agent, provided the patient does not have significant LV dysfunction or symptoms of congestive heart failure.
ANTIPLATELET THERAPY
Aspirin. Aspirin inhibits the synthesis of thromboxane A2, resulting in irreversible inhibition of the COX-1-mediated platelet aggregation for the life of a platelet. In a study of patients with UA, the use of aspirin resulted in a 51% reduction in risk of cardiac death or nonfatal infarction at a mean follow-up of 18 months.23 As such, aspirin has since become part of the standard therapies used for all patients presenting to the hospital with NSTE-ACS. Aspirin should be given as an initial dose of 325 mg, followed by maintenance doses of 75 to 162 mg daily. If a patient undergoes percutaneous coronary intervention with stent placement, then doses of 162 to 325 mg may be considered post procedure. Aspirin allergy is rare and anyone reporting an allergy to aspirin should be further questioned as to the validity of this statement. In patients with true hypersensitivity to aspirin (hives or angioedema) alternative agents including clopidogrel may be substituted. Alternately, aspirin desensitization may be performed.
P2Y12 receptor blockers. The P2Y12 receptor antagonists include the thienopyridines (clopidogrel, prasugrel, and ticlopidine) that cause platelet inhibition by actively binding to the P2Y12 receptors of platelets resulting in irreversible inhibition of ADP-mediated platelet aggregation and activation for the life of the platelet, and ticagrelor that reversibly binds to the P2Y12 receptor on the surface of platelets. These agents differ slightly in their pharmacokinetics, and side effects; therefore, careful consideration should be given to a patient’s clinical characteristics when choosing one agent over another. Ticlopidine was the first agent available for use and has been shown to be of benefit in the secondary prevention of stroke or MI. However, ticlopidine has the potential for serious hematologic side effects including neutropenia, thrombotic thrombocytopenia purpura, and bone marrow aplasia. Given the availability of several other alternative agents, ticlopidine is seldom used in modern practice. The remainder of this section will focus on a review of the more commonly used agents.
Dual antiplatelet therapy with clopidogrel plus aspirin is now standard practice for the treatment of patients presenting to the hospital with NSTE-ACS; this was largely based on the results of the Clopidogrel to Prevent Recurrent Events (CURE) trial.24 In this study, 12,562 patients with NST-ACS were randomized to 300 mg oral load of clopidogrel followed by 75 mg daily in addition to aspirin or aspirin alone. Patients were followed up for 3 to 12 months. The primary outcome, which was a composite of death from cardiovascular causes, nonfatal MI, or stroke was significantly lower in the clopidogrel group compared to the placebo group (relative risk with clopidogrel as compared with placebo, 0.80; 95% CI, 0.72 to 0.90; P < .001). In this study, the group of patients assigned to clopidogrel had a significantly higher rate of major bleeding when compared with those in the placebo group although life-threatening bleeding or hemorrhagic stroke was similar in the two groups. Increased bleeding was notable among the patients undergoing urgent CABG surgery. Therefore, although there is a clear benefit to the routine use of dual antiplatelet therapy for patients with NSTE-ACS, one must exercise caution in patients who are at high bleeding risk, or among those patients in whom urgent cardiac surgery is planned. If cardiac surgery is planned, the ACC/AHA guidelines recommend withholding clopidogrel for 5 days prior to elective surgery if possible.4
Unlike aspirin, there is a delayed onset of optimal platelet inhibition following clopidogrel dosing; this varies with each person and with different doses, and can be as late as 12 to 24 hours. Studies have suggested shorter time to optimal platelet inhibition with a larger loading dose,25 although the optimal loading dose of clopidogrel is not well established. In a recent clinical study examining this question,26 patients with ACS were randomly assigned to one of two treatment strategies including a loading dose of 600 mg of clopidogrel followed by 150 mg daily for 1 week and then 75 mg daily thereafter, or a loading dose of
300 mg followed by 75 mg daily. In this trial there was no difference in cardiovascular death, MI, or stroke at 30 days for either of the two treatment strategies. However, an analysis examining outcome only among those patients who underwent percutaneous coronary intervention demonstrated a 32% reduction in the incidence of stent thrombosis in the double dose clopidogrel group (Hazard ratio 0.68, 95% CI 0.55 to 0.85, P = .001). Major bleeding was significantly higher in the double dose clopidogrel group. Given this information, consideration may be given to a 600 mg loading dose and 150 mg daily initial dose of Clopidogrel for the first week for patients with ACS who undergo percutaneous coronary intervention; there appears to be little added benefit to double dose therapy of clopidogrel among NSTE-ACS who are medically treated or who require surgical intervention.
300 mg followed by 75 mg daily. In this trial there was no difference in cardiovascular death, MI, or stroke at 30 days for either of the two treatment strategies. However, an analysis examining outcome only among those patients who underwent percutaneous coronary intervention demonstrated a 32% reduction in the incidence of stent thrombosis in the double dose clopidogrel group (Hazard ratio 0.68, 95% CI 0.55 to 0.85, P = .001). Major bleeding was significantly higher in the double dose clopidogrel group. Given this information, consideration may be given to a 600 mg loading dose and 150 mg daily initial dose of Clopidogrel for the first week for patients with ACS who undergo percutaneous coronary intervention; there appears to be little added benefit to double dose therapy of clopidogrel among NSTE-ACS who are medically treated or who require surgical intervention.
Clopidogrel is a pro-drug requiring conversion to an active metabolite by the hepatic cytochrome P450 isoenzymes to exhibit antiplatelet effects. It has been shown that certain genetic subtypes have a loss of function of the allele of the CYP2C19 gene (encoding for one of the hepatic isoenzymes responsible for the metabolism of clopidogrel) resulting in reduced conversion of clopidogrel to its active metabolite. Many studies have suggested that patients with genetic variants of this gene have a reduced pharmacodynamic response to clopidogrel and a higher rate of cardiovascular events when compared with those patients with normal genetic subtypes.27,28,29 and 30




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree