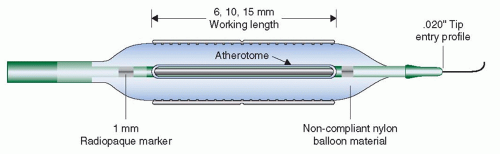
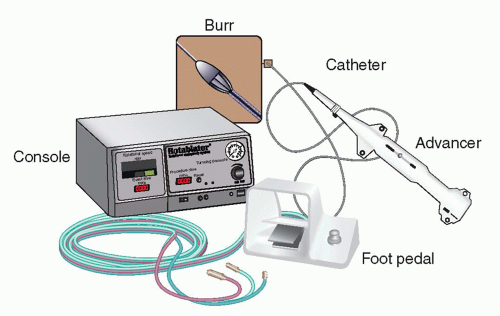
The studies evaluating the clinical efficacy and safety of rotational atherectomy were performed in the late 1990s (see
Fig. 13-9). The STRATAS trial compared the strategies of routine debulking (burr-to-artery ratio of 0.6-0.8 with adjunctive percutaneous coronary angioplasty [PTCA]) versus that of aggressive debulking (burr-to-artery ratio of 0.7-0.9 with no- or low-pressure PTCA), and observed no difference in procedural outcomes or 9-month angiographic restenosis or target lesion revascularization (
2). Rotational atherectomy was compared with PTCA in the randomized COBRA and DART trials, comparing clinical outcomes in de novo coronary artery lesions (
3,
4). Efficacy endpoints, including rates of restenosis, were similar between the two techniques, with significant higher incidence of no reflow after rotational atherectomy compared with PTCA. In the randomized ERBAC trial comparing PTCA, rotational atherectomy, and laser atherectomy, restenosis was actually higher for rotational atherectomy than for balloon angioplasty, and was associated with higher rates of complication (
5). In the ARTIST trial, rotational atherectomy was shown to have higher restenosis and major adverse cardiac events (MACE) for in-stent restenotic lesions compared with PTCA (
6). As a result of these studies, rotational atherectomy was shown to be at most noninferior to PTCA with respect to restenosis, and associated with higher rates of adverse cardiac events (
Table 13-6).
With the advent of the stent era, continued interest in debulking prior to stent placement led to the evaluation of atherectomy as an adjunct to stenting. In the SPORT trial, patients with moderately to heavily calcified lesions were randomized to either rotational atherectomy followed by stenting or PTCA followed by stenting (
7). The trial was stopped early, but the available data were presented, which showed that the primary endpoint of restenosis at 9 months was similar between the two groups, whereas MACE were higher with rotational atherectomy.
As a result of these clinical trials and the 2011 update of percutaneous coronary intervention (PCI) guidelines (Table 13-7),
coronary atherectomy is given a Class IIA recommendation for the preparation of fibrotic or heavily calcified lesions that might not be crossed by a balloon catheter or adequately dilated prior-to-stent implantation (level of evidence: C). Coronary atherectomy was given a Class III indication (harm) in the routine treatment of de novo or in-stent restenosis (level of evidence: A) (
8).