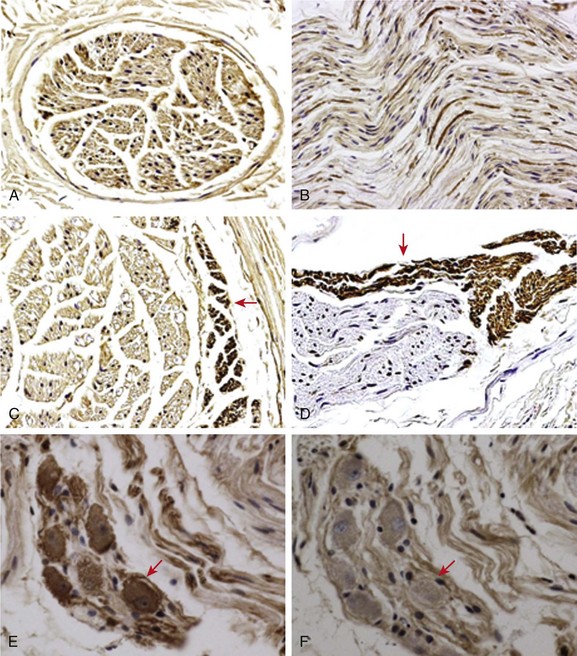
40 In addition to the extrinsic cardiac nerves, the heart is also richly innervated by an extensive intrinsic cardiac nervous system.1,2 The intrinsic cardiac nervous system includes sensory, interconnecting, and autonomic neurons that communicate with each other and with the extrinsic cardiac nervous system. The nerve structures of the intrinsic cardiac nerves are found in various parts of the heart, but mostly in the ganglionated plexi within epicardial fat pads. Among the ganglionated plexi, the right-atrial ganglionated plexi innervates the sinus node, whereas the inferior vena cava–inferior atrial ganglionated plexi (at the junction of inferior vena cava and the left atrium) innervates the atrioventricular node. Another region that is richly innervated is located at the pulmonary vein (PV)-left atrium (LA) junction. Radiofrequency catheter ablation at these sites can potentially result in successful denervation and prevent the inducibility of AF.3 However, preserving (rather than ablating) the anterior epicardial fat pad during coronary arterial bypass surgery decreases incidence of postoperative atrial fibrillation. A common misunderstanding of the autonomic nervous system is that the nerve structures are either sympathetic or parasympathetic. For example, the term vagal tone is generally used to describe the level of activity in the parasympathetic nervous system. Vagal denervation was used to describe the successful elimination of bradycardiac responses during catheter ablation of AF. The fact, however, is that the vagus nerves and almost all other cardiac nerve structures contain both sympathetic (adrenergic) and parasympathetic (cholinergic) components. It is not possible to stimulate or ablate one branch of the autonomic nervous system without affecting the other. Tan et al4 performed immunostaining of tissues from the human PV-LA junction. The authors found that adrenergic and cholinergic nerves coexist in all ganglionated plexi. It is also possible for the same neuron to express both TH and ChAT. These findings indicate that it is impossible to target either sympathetic or parasympathetic nerves selectively during radiofrequency catheter-ablation procedures. Sympathetic nerve fibers are also present in the thoracic vagus nerve.5 More recently, Park et al.6 performed TH and ChAT staining of the left cervical vagus nerve (Figure 40-1). ChAT positive nerve structures formed a majority of the cervical vagus nerve (see Figure 40-1, A, B). However, a small amount of TH-positive nerves were also present at the edge of the nerve bundles (see Figure 40-1, C, D). Unexpectedly, the authors identified sympathetic neurons in the vagus nerve (see Figure 40-1, E), indicating that the cervical vagus nerve was a source of sympathetic innervation. The same neurons stained negative for ChAT (see Figure 40-1, F). The presence of both TH-positive neurons and TH-positive nerve fibers is consistent with the notion that the vagus nerve is a mixed sympathetic and parasympathetic nerve structure. Therefore, vagal tone includes both sympathetic and parasympathetic components. Figure 40-1 Immunocytochemical staining of the cervical vagus nerve. A and C, Examples of the nerves sectioned transversely. Other panels show nerves sectioned longitudinally (craniocaudal). Each cervical vagus nerve contains multiple parallel nerve bundles, most staining positively (brown) for cholineacetyltransferase (ChAT), as shown in A and B. However, a small percentage of the nerve bundles, primarily at the periphery of the nerves, stained positively for tyrosine hydroxylase (TH; arrows in C and D). E, In addition, TH-positive ganglion cells are also present in the nerves. F, These cells were ChAT negative. These findings indicate that bundles of sympathetic nerves are present within the cervical vagus nerves. The presence of sympathetic ganglion cells in the vagus nerve suggests that the cervical vagus nerve is also an important source of sympathetic innervation. A to C, Original magnification ×100. D to F, Original magnification ×40. (From Park HW, Shen MJ, Han S, et al: Neural control of ventricular rate in ambulatory dogs with pacing induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol 5:571–580, 2012.) Jung et al7 continuously recorded the activity of stellate ganglia in healthy dogs for an average of 41.5 days and documented that both the heart rate and the stellate ganglion nerve activity (SGNA) showed a circadian variation. Ogawa et al5 and Tan et al8 then applied the same methods to record vagus nerve activity (VNA). These earlier studies showed several findings about nerve discharges that were previously unknown. First of all, there are fundamentally two different types of nerve activities (Figure 40-2). The vast majority of the nerve activities were the low-amplitude burst discharge activities (LABDA), with an amplitude less than 0.2 mV and variable duration. A second type of nerve activity is the high-amplitude spike discharge activity (HASDA) with amplitude of greater than 0.2 mV (average, 1.4 mV). There is usually a nearly isoelectric interval between the spikes, with obvious depolarization shifts in some of the episodes. The HASDA has a frequency of approximately 6.6 Hz, and there is an average of 6.7 spikes per run.5 HASDA episodes were rare, with an average of approximately 15 episodes per 24 hours. However, when they were observed, there was a high likelihood of both atrial and ventricular arrhythmias.5,9,10 Figure 40-2, A, shows examples of LABDA and HASDA in a normal dog. Note that LABDA in the SGNA can accelerate the heart rate. HASDA usually occurs during LABDA and can further accelerate the heart rate. Figure 40-2, B, shows that a HASDA episode immediately precedes the premature atrial contraction in a dog with pacing-induced heart failure.5 In another study, we have observed multiple episodes of HASDA-induced premature ventricular contractions and nonsustained ventricular tachycardia.9 A second important observation is that, in ambulatory animals, the nerve structures often activate either simultaneously or alternatively, suggesting a close coordination among the nerve activities from different parts of the autonomic nervous system. For example, the left and right stellate ganglion usually activate together.7 Similarly, the VNA can activate with SGNA.5,8 The VNA can also activate simultaneously with the ganglionated plexi.11–13 Figure 40-3, A, shows simultaneous discharges of the right and left stellate ganglion in a normal dog. Figure 40-3, B, shows simultaneous recording of both extrinsic and intrinsic nerve activities in a dog with intermittent rapid atrial pacing. Note that both extrinsic nerve activities (SGNA and VNA) activated together with one of the intrinsic nerve structure (superior left ganglionated plexus, SLGPNA), but not the ligament of Marshall ganglionated plexus. The VNA and SLGPNA activation patterns were almost mirror images of each other, suggesting that these two structures closely coordinate with each other. Another important finding is that the SGNA in Figure 40-3, A, resulted in less apparent heart rate acceleration than that shown in Figure 40-2, whereas in Figure 40-3, B, there was sinus rate acceleration associated with SGNA. Subsequent studies showed that right anterior ganglionated plexus has an important role in heart rate control.13 Therefore, recording extrinsic nerve activity alone might not be sufficient in determining the mechanisms of heart rate control in ambulatory animals. The complex interactions among different autonomic nerve structures is one of the mechanisms by which heart rate variability measurements in general fail to accurately predict the instantaneous sympathetic and parasympathetic nerve activities. In dogs with heart failure, the relationship between nerve discharge and heart rate control is further uncoupled because of the sinus node dysfunction.13
Neural Activity and Atrial Tachyarrhythmias
Cardiac Nerves
Intrinsic Cardiac Nerves
Coexistence of Sympathetic and Parasympathetic Nerves in the Same Structure
Neural Activity and Atrial Tachyarrhythmias
Recording Neural Activities in Ambulatory Animals
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neural Activity and Atrial Tachyarrhythmias
