INTRODUCTION
Learning Objectives
The student will be able to describe the clinical features of the neonatal respiratory distress syndrome (RDS) and how those features relate to the physiology of increased alveolar surface tension.
The student will be able to provide the scientific bases of medical therapies for neonatal RDS including prenatal steroids, continuous positive airway pressure, mechanical ventilation, and exogenous surfactant.
The student will be able to characterize the diving response and laryngeal chemoreceptor reflex as used to study the sudden infant death syndrome (SIDS).
NEONATAL RESPIRATORY DISTRESS SYNDROME (RDS)
Normal intrauterine lung development is comprised of stages featuring both cellular proliferation to increase total lung size, and cellular differentiation by which the airway and alveolar architectures are elaborated from primordial organ buds (Chaps. 2 and 37). By the time the fetus reaches normal term gestation (~38 weeks), there is ongoing alveolarization and normally the lungs are structurally and biochemically prepared for the transition to extrauterine function in gas exchange. Among the most profound changes in the lung at the time of birth are: (1) the replacement of fluid with gas in the airways, with consequent generation of alveolar surface tension forces that must be overcome; and (2) a fall in pulmonary vascular resistance, with consequent increase in pulmonary blood flow. These changes must occur over a relatively short time frame for normal pulmonary function. As an additional challenge, the newborn’s lungs are surrounded by ribs and related thoracic structures that are considerably more compliant than in the adult, owing to their incomplete ossification. This greater thoracic compliance at birth limits the expansive traction that can be exerted by the chest wall on the lungs’ visceral pleura, and thus alters the inherent equilibrium that defines FRC and the work of breathing (Chap. 6). Given these challenges, it is no surprise that respiratory failure is a major contributor to newborn morbidity and mortality.
By far, the most common cause of life-threatening respiratory disease in newborns is prematurity. Insufficient development and maturation of the type 2 surfactant-secreting epithelial cells leaves the preterm lung unprepared to overcome surface tension forces at the alveolar gas-fluid interface, as described by the law of Laplace (Chap. 5). The consequences of surfactant insufficiency in the preterm lung and the clinical treatment to overcome these consequences will be discussed in depth here. As these are elaborated below, the student should remember that surfactant dysfunction contributes to the pathogenesis of lung disease beyond the immediate postnatal period, such as ALI and ARDS (Chap. 28), emphasizing the broader implications of the concepts outlined in this chapter.
Before discussing the pathophysiology of RDS and the current modalities of treatment, it is useful first to consider why the historical mode of treatment, administration of oxygen, was by itself unsuccessful. Doing so will emphasize the particular consequences of diffuse alveolar collapse in the generation of intrapulmonary shunting. It will also emphasize consequences of open fetal cardiovascular channels to the generation of extrapulmonary shunting.
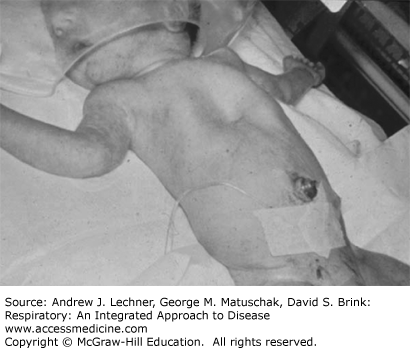
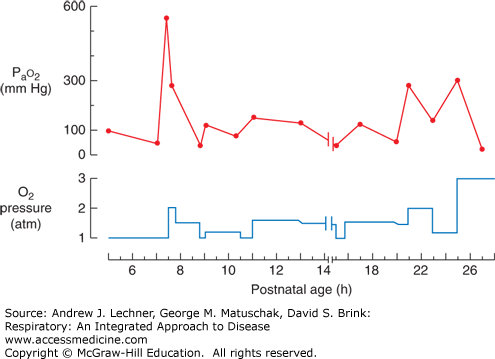
The hypoxemia seen in preterm newborn infants with RDS can be only partially and transiently overcome by placing the infant in high FIO2, such as that provided by an oxygen hood (Fig. 39.1). In more severe cases, the situation continues to worsen to a point at which even 100% O2 cannot maintain normoxemia. The most dramatic example of the failure of O2 therapy alone was the heroic effort to treat preterm infants in the early 1960s with hyperbaric oxygen. In order to increase O2 delivery to the disease lungs beyond that supplied at atmospheric pressure, preterm infants with severe RDS were placed in a hyperbaric chamber. One infant treated in this manner was Patrick Bouvier Kennedy, son of then President John F. Kennedy of the United States. Unfortunately, the benefits of hyperbaric oxygen were transient at best in each of the reported infants (Fig. 39.2). Ultimately all of the reported infants in which hyperbaric oxygen was used, including Patrick Kennedy, died of hypoxemia.
FIGURE 39.2
Response of a neonate’s Pao2 to exposure to O2 and to increased atmospheric pressure in a hyperbaric chamber. Note the increasingly aggressive use of multiple atmospheres of pure O2 and the only transient responses in Pao2 that resulted. Adapted from Cochran WD: A clinical trial of high oxygen pressure for the respiratory distress syndrome, N Engl J Med Feb 18;272:347-51, 1965.
Unfortunately, the unstable and deteriorating clinical course of Patrick Kennedy was all too common in an era when methods to intervene successfully in RDS had not yet been developed. In subsequent decades, a vast amount of new information about the nature of pulmonary surfactant and the developmental biology of the type 2 cell emerged. These included an understanding of the timeline of their maturation and the normal composition of surfactant phospholipids and their associated proteins (Chaps. 5 and 10). The primary abnormality in neonatal RDS is a developmental deficiency in the production or secretion of pulmonary surfactant. The physical, laboratory, radiographic, and pathologic findings in neonatal RDS (Table 39.1) can each be understood based on the pivotal physiologic role of pulmonary surfactant (see Chaps. 5, 15, and 16). It is important to emphasize that neonatal RDS is not the same condition as the acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) discussed in Chap. 28. However, abnormalities of the surfactant system can contribute to the pathogenesis of both types of RDS, and there is considerable overlap in the clinical findings.
| Physical | Laboratory | Radiographic | Pathologic |
|---|---|---|---|
| Tachypnea | ↓ Pao2, and if severe, ↑ Paco2 | ↓ Lung volume | Hyaline membranes |
| Grunting | “Ground glass” opacities | Atelectatic distal airways | |
| Sternal retractions | Air bronchograms | Underinflation | |
| Nasal flaring | |||
Recall from Chap. 5 that lung inflation and deflation can be modeled as a bubble at the end of a straw, whose expansion and contraction are governed by forces described by the law of Laplace. From this physiological principle, several consequences follow:
In the absence of a substance to lower surface tension forces, smaller alveoli collapse into larger ones;
Positive airway pressure applied during inspiration inflates such lungs to only small volumes with high critical opening pressures, (PCO); and
Any open lung units are prone to premature collapse toward very low lung volumes during expiration.
Much of what presents as neonatal RDS in a premature infant follows as the predictable consequences of these principles, and can initiate or synergize with defective or suboptimal performances by other organ systems, including the heart.
A number of specific factors have been identified that increase or decrease the likelihood of an infant developing RDS (Table 39.2). It is important to emphasize that a single risk factor such as prematurity is not 100% predictive, in part because it may be offset by factors like female gender, vaginal delivery, or prenatal glucocorticoids that reduce risk. These will be discussed in subsequent sections of this chapter.
As a cellular and biochemical process, surfactant synthesis is increased by glucocorticoids, thyroid hormone, and thyroid releasing hormone, but is decreased by increased levels of insulin or androgens. Surfactant secretion also is increased by β-adrenergic agonists and by endogenous catecholamines released during the physical stress of labor itself. Due to these and other variables (Table 39.2), not every preterm infant develops RDS while some infants of relatively late gestation do.
Because patients with RDS move less air than normal with each inspiratory effort, that is, VT is less, their respiratory frequency f must increase to maintain V̇E. This extra work of breathing may eventually fatigue neonates, at which point their Pao2 falls and Paco2 may rise. Episodic apnea is also common in infants with RDS.
A patient with RDS reflexively attempts to overcome the tendency for alveoli to collapse during expiration by partially closing the glottis to create an air stent (see Chaps. 25 and 30). The increased PAW generated by expiring against a partially closed glottis stabilizes alveoli. Then as the patient opens the glottis to complete expiration and begin inspiration, the increased PAW is suddenly released and causes an audible grunt. Students may visit Web site http://rale.ca/grunting.htm to hear a recording of grunting.
Patients with RDS must generate more negative PIP to move an equivalent volume of air into lungs that are less compliant (see Fig. 5.2). A large negative PIP retracts inwardly the unossified chest wall during inspiration. While sternal and thoracic retractions can be seen in many infants, they are particularly prominent among premature ones whose chest walls are excessively compliant (Fig. 39.1).
The nares are prone to inward collapse during inspiration if their patency is not maintained by contraction of the alea nares muscles. The neonate with RDS attempts to minimize nasal resistance to airflow during labored inspiration by reflexively contracting these muscles and thus presents with the clinical finding of nasal flaring.
PaO2 and/or SaO2 fall in infants with RDS for the same reasons that adults develop hypoxemia (Chaps. 8, 9, and 28). These are summarized in Table 39.3, and as for ARDS can be categorized by their responsiveness to only an increase in FIO2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree