Chapter 32 Natural History and Nonoperative Treatment of Chronic Lower Extremity Ischemia
Although only 1% to 2% of people younger than 50 years suffer from symptoms of intermittent claudication, this figure rises to 5% in those aged 50 to 70 years and to 10% in those older than 70.1–3 It is estimated that 8 to 12 million people in the United States,4 and 27 million people in North America and Europe,5 suffer from peripheral arterial disease (PAD), and this number is expected to rise as the population ages.
Stratification and Epidemiology
Chronic lower extremity ischemia represents a clinical spectrum. Clinical manifestations range from asymptomatic disease and atypical symptoms to classic intermittent claudication and critical limb ischemia with impending tissue loss. Intermittent claudication is typically reported as the number of blocks a patient can walk on level ground at a normal speed without having to stop; however, patients are frequently poor judges of objective walking distance. Pharmaceutical trials have stratified patients based on walking distances (initial or absolute claudication distances) or claudication times during either fixed or graded load treadmill testing. Recently, tools such as the Walking Impairment Questionnaire have assisted in the stratification of intermittent claudication.6 Combining the objective measurements of ischemia (ankle-brachial pressure index, toe pressure, pulse-volume recordings) with the clinical situation helps to define the natural history of various patient groups with chronic lower extremity ischemia.
Lower extremity PAD is an independent risk factor for cardiovascular morbidity and mortality. Atherosclerotic cardiovascular disease is a systemic process affecting multiple arterial beds, including the coronary, cerebrovascular, upper and lower extremities, and visceral arteries. There is often significant disease overlap in the various arterial segments.7,8 A number of large, population-based epidemiologic studies have reported on the incidence and prevalence of PAD. PAD incidence and prevalence is dependent on the definition of PAD used.
Asymptomatic Arterial Insufficiency
Asymptomatic PAD is defined as a decreased ankle-brachial index (ABI) without lower extremity symptoms. Most studies use an ABI of less than 0.9 as a reference standard for PAD.5 The presence of asymptomatic lower extremity occlusive disease varies, but available data indicate that for every patient with intermittent claudication, there are probably three others with similar disease who do not complain of symptoms or have atypical symptoms.9 Ratios of symptomatic to asymptomatic patients range from 1 : 1.8 to 1 : 5.3.1,2,10,11
The prevalence of asymptomatic PAD was 25.5% among 1537 participants in the Systolic Hypertension in the Elderly Program.12 Data from a nationwide cross-sectional study, based on more than 350 primary care practices, demonstrated that 13% of 6979 patients older than 50 years had abnormal ABIs, with or without symptoms of intermittent claudication.7 Only 24% of patients with chronic lower extremity ischemia had a previous diagnosis of PAD. Asymptomatic patients accounted for 48% of newly diagnosed PAD.
Incidence of Symptomatic Peripheral Arterial Disease
The majority of epidemiologic studies of PAD have focused on patients with intermittent claudication, defined as leg pain with walking (most often calf pain, but it can involve the thighs and buttocks as well). Pain is induced by exercise and is relieved by rest. The reported incidence of PAD varies between 2.2% of a population aged 33 to 82 years and 17% of a population aged 55 to 70 years.1,2 In the Framingham Heart Study, the incidence of PAD was based on symptoms of intermittent claudication in subjects 29 to 62 years old. The annual incidence of intermittent claudication per 10,000 subjects at risk rose from 6 in men and 3 in women aged 30 to 44 years to 61 in men and 54 in women aged 65 to 74 years.13 The average rate of development of intermittent claudication over a 2-year period in subjects older than age 50 was 0.7% in men and 0.4% in women.13
In the Edinburgh Artery Study of almost 1600 subjects older than 55 years, the 5-year cumulative incidence of PAD was 9%.14 Bowlin and coworkers15 followed 8343 Israeli men over a 21-year period and found a cumulative incidence of 43.1 per 1000 population. In the Quebec Cardiovascular Study of 4570 men followed over 12 years, an incidence of 41 per 10,000 population per year was noted.16 In the large prospective Physicians’ Health Study, 433 incident cases of PAD were reported among 22,071 relatively healthy men.17
Prevalence of Symptomatic Peripheral Arterial Disease
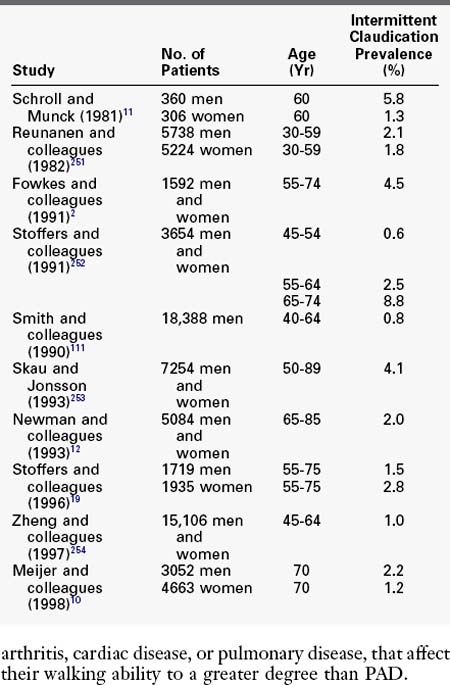
Epidemiologic studies have used both questionnaires and noninvasive vascular laboratory screening to estimate the prevalence of PAD in the elderly adult population. Before the development of reliable noninvasive testing, the diagnosis of PAD was based on standardized patient questionnaires. Among questionnaires, the World Health Organization/Rose Questionnaire and the Edinburgh Classification Questionnaire (ECQ) have been the most extensively studied. The ECQ appears to be more robust, with a sensitivity of 91% and a specificity of 99% for the diagnosis of intermittent claudication.18 Using the ECQ, the prevalence of lower extremity arterial disease was estimated to be 4.6% in the Edinburgh Artery Study in men and women between the ages of 55 and 74 years.2 Other studies have found the prevalence of intermittent claudication in adults older than age 45 to be approximately 1% to 5% (Table 32-1).
TABLE 32-1 Prevalence of Intermittent Claudication by History or Questionnaire in Large Population Studies
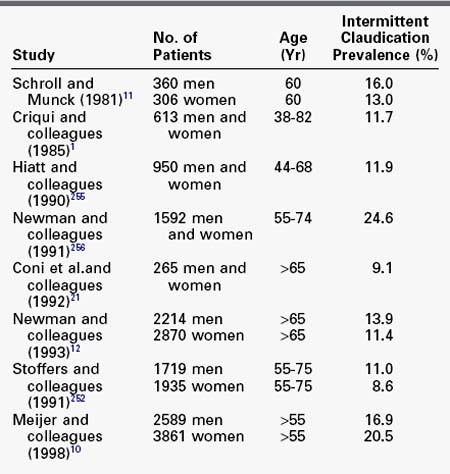
When ABI is used as a reference standard, the detected prevalence of lower extremity arterial occlusive disease is even greater, likely because of the inclusion of patients who are asymptomatic or have atypical symptoms. The overall age-adjusted prevalence of PAD diagnosed on the basis of the ABI is approximately 12%; for intermittent claudication, it is 1% to 2% up to age 50 and 5% to 7% from the seventh decade onward.19 In the Cardiovascular Health Study, the prevalence of a decreased ABI (<0.9) was 12.4% in adults aged 65 years or older in four U.S. communities.12 Using the same criteria, investigators in the Rotterdam Study reported a PAD prevalence of 19% in subjects older than 55 years.10 In the Edinburgh Artery Study, the prevalence of lower extremity arterial occlusive disease diagnosed using ABI was 17% in subjects between the ages of 55 and 74 years.2 In a Danish study, the prevalence of lower extremity arterial occlusive disease in 60-year-old subjects was 16% for men and 13% for women (Table 32-2).20 Thus, objective standards of measurement identify a greater number of patients with PAD than does reliance on patient description of symptoms.
TABLE 32-2 Prevalence of Peripheral Arterial Disease Based on Ankle-Brachial Index Abnormalities in Large Population Studies
Several factors might explain the lack of sensitivity of questionnaires and the increased prevalence of PAD with noninvasive testing.2,21 First, symptoms might not occur until the disease is advanced. This is particularly relevant in older patients who may rarely walk more than one or two blocks at a time in the performance of their activities of daily living, or who may assume that leg pain while walking is a natural part of the aging process. Second, patients with PAD may have other comorbidities, such as arthritis, cardiac disease, or pulmonary disease, that affect their walking ability to a greater degree than PAD.
Risk Factors
Smoking
The specific mechanisms by which tobacco exerts its adverse effects on arteries remain poorly understood; however, a direct relationship between tobacco smoking and peripheral vascular disease has been well established.22 All epidemiologic studies of lower extremity arterial disease have confirmed cigarette smoking as a strong risk factor for the development of such disease, with relative risk ratios ranging from 1.7 to 7.5.1,10,12,23–27 A case-control study revealed a sevenfold higher risk of developing PAD in ex-smokers compared with those who had never smoked, and the risk increased to sixteenfold in current smokers compared with those who had never smoked.28 The diagnosis of lower extremity arterial disease is made up to a decade earlier in smokers compared with nonsmokers. More than 90% of all patients referred to vascular clinics for PAD have a history of smoking.29
In addition to the chronic effects of smoking on the development of atherosclerosis, smoking has acute effects on lower extremity function. Smoking two cigarettes within a 10-minute period resulted in an acute lowering of the ABI in chronic smokers from 0.64 ± 0.14 to 0.55 ± 0.11 (p = 0.008).30 In addition to having adverse influences on atherosclerosis, the carbon monoxide in tobacco smoke may directly contribute to claudication. Smoking is associated with acute drops in treadmill walking distances, presumably owing to carbon monoxide.31 An immediate and significant decrease in the time or distance that patients can walk on the treadmill before they get claudication symptoms has been demonstrated when air containing carbon monoxide is breathed.31,32 Smokers have an increased risk of peripheral vascular disease progression,33 myocardial infarction, stroke, and death.34 Smokers also have an increased risk of major amputation.35,36
Diabetes Mellitus
A strong association exists between diabetes mellitus and PAD. Two types of vascular disease are seen in patients with diabetes: microcirculatory dysfunction involving the capillaries and arterioles of the kidneys, retina, and peripheral nerves, and a macroangiopathy involving the peripheral and coronary arterial circulation.37 The Framingham Study was one of the first major epidemiologic studies to demonstrate the association between diabetes and PAD. Diabetes increased the risk of claudication by a factor of 3.5 in men and 8.6 in women.38 Numerous subsequent studies have associated impaired glucose tolerance with a twofold to fourfold increase in the risk of developing intermittent claudication.39–44 In an elderly white population, 20.9% of patients with diabetes mellitus and 15.1% of patients with an abnormal glucose tolerance test had an ABI less than 0.9.45 In a Swedish study, 21% of patients with diabetes had signs of PAD.46 The duration and severity of diabetes mellitus correlate strongly with the incidence and severity of PAD.
Patients with diabetes mellitus often develop symptomatic forms of PAD and have poorer lower extremity function than do those with PAD alone.47 The prevalence of diabetes in patients undergoing lower extremity revascularization ranges from 25% to 50%, compared with a prevalence of 6% in the general population.48 The rate of lower extremity amputation is sevenfold to tenfold higher in diabetic patients than in those without diabetes.23,49–51 In fact, diabetes leads to most of the nontraumatic lower extremity amputations in the United States. In addition to diabetes, insulin resistance and hyperinsulinemia are also risk factors for PAD.26,52
Gender
Early epidemiologic studies focused on the prevalence of PAD in men. The popular notion based on the Framingham Study was that symptomatic PAD in women lagged behind men by 10 years53 and that women were generally not affected by PAD until after menopause. However, more recent epidemiologic studies indicate that PAD prevalence and incidence in men and women are similar. Several studies have demonstrated that the age-adjusted incidence of intermittent claudication is equal in both genders,20,54 with the frequency of PAD among diabetic women markedly higher than that among diabetic men.20 Among subjects with a low ABI, coronary artery disease was less prevalent among women. Women also had a lower frequency of cerebrovascular disease.54 In another study, the prevalence of PAD was almost identical in men and women; however, other cardiovascular disease was twice as prevalent in men.7 Progression of PAD, as measured by changes in ABI, appears to be the same in men and women.55 However, women may suffer faster functional decline. A prospective trial of 380 men and women showed that at 4-year follow-up, women were more likely do have a decline in walking distance and more likely to develop mobility related disability than men. These functional differences seem to be attributable to smaller baseline calf muscle area in women.56 Epidemiologic studies have shown that women may be more susceptible to aortoiliac arterial occlusive disease than men are.57 Autopsy findings also provide important information about gender differences in the occurrence of atherosclerotic changes in various arterial beds. Compared with men, women have a greater extent of fatty streaks in the abdominal aorta, but not in the coronary arteries.58
It is possible that PAD is underdiagnosed in women to a greater degree than in men. Studies have shown that women are less likely than men to have diagnosed PAD on the basis of symptoms, even if clinically significant PAD is present on noninvasive examinations.59,60 In addition, it has been shown that infrainguinal arterial reconstructions performed on women tend to be for more advanced disease compared with men, and the women tend to be older.61,62 The reasons for the more advanced presentation in women are unclear. It has been speculated that because women more frequently assume a caretaker role, they are more likely to ignore their own medical care; or perhaps women are more likely to ignore mild to moderate pain, attributing it to a consequence of old age.63 It is clear that the previous dictum of PAD being primarily a disease of men is changing as more data about its effect on women emerge.
Race
Few studies have assessed differences in PAD prevalence among different ethnic groups. One early study indicated that African American patients with PAD typically had a higher occurrence of infrapopliteal atherosclerosis, which was associated with a greater incidence of limb loss.64 Certain studies also indicate that PAD may be underreported in the African American population. In the Atherosclerosis Risk in Communities Study, more than 4000 African Americans were screened for PAD. Despite a greater prevalence of hypertension and diabetes, the prevalence of PAD measured by questionnaire was lower among African American men than among white men. However, 3.3% of African Americans had an ABI less than 0.9, compared with only 2.3% of whites.54 In the Cardiovascular Health Study, the nonwhite population tested had a 3.5-fold increased frequency of an ABI less than 0.8.12
Hyperlipidemia
It is estimated that up to 50% of patients with lower extremity arterial disease have hyperlipidemia. In the Framingham Study, a fasting cholesterol level greater than 270 mg/dL was associated with a doubling of the incidence of intermittent claudication.53 Population studies have demonstrated that the relative risk of PAD is 2.05 in patients with hypercholesterolemia,65 1.7 in patients with hypertriglyceridemia,24 and 2.0 in patients with elevated levels of lipoprotein (a).66 Other studies have suggested that triglyceride levels are not an independent risk factor for PAD when corrected for other serum lipid variables.24,67
Ridker and colleagues68 evaluated multiple plasma lipid constituents, including total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, lipoprotein (a), and apolipoproteins A-I and B-100, and the risk of developing PAD. Of the lipid markers tested, the ratio of total cholesterol to HDL cholesterol was the strongest predictor of PAD development, and the addition of screening for other lipid abnormalities did not improve predictive values. The addition of screening for two nonlipid variables—C-reactive protein and fibrinogen—did, however, improve the prediction of PAD risk.
Hyperhomocysteinemia
A number of prospective and retrospective studies have suggested an association between elevated levels of plasma homocysteine and premature vascular disease in the coronary, cerebrovascular, and peripheral circulation.69–72 Early studies suggesting this association, however, were based on small numbers of patients. Darius and colleagues73 evaluated plasma homocysteine levels as an independent risk factor for PAD in 6880 primary care patients older than 65 years. Although PAD (defined as an ABI < 0.9) was more frequently diagnosed in patients in the highest quintile of homocysteine levels (24.3%) than in the lowest quintile (13.0%; crude odds ratio 2.1), the association was less strong after adjusting for other atherosclerotic risk factors (odds ratio 1.4). Thus, the association between hyperhomocysteinemia and atherosclerosis is likely mild.
Serum Markers
Fibrinogen and C-reactive protein have been implicated in the pathogenesis of PAD in numerous studies.34,74–79 The role of these factors in the pathogenesis of atherosclerosis is unclear; however, they are thought to be potential markers of endothelial dysfunction.80 Elevated fibrinogen levels were independent risk factors for the development of PAD in both the Edinburgh and Rotterdam studies.10,81
C-reactive protein is an acute phase reactant that is elevated in acute inflammatory conditions. Persistent elevations are observed in chronic inflammatory disorders. The elevation of this factor in patients with atherosclerosis has led to the theory that inflammation contributes to the development of atherosclerosis. Whether C-reactive protein itself causes atherosclerosis is unknown. Other atherogenic risk factors such as age,82,83 smoking,84 diabetes,85 and hyperlipidemia86 are associated with elevated levels of C-reactive protein in the absence of PAD. Therefore elevated C-reactive protein may be an epiphenomenon associated with, but not causative of, an atherogenic state.
McDermott and colleagues87 evaluated the association of elevated inflammatory biomarkers and physical performance in patients with PAD. Both elevated C-reactive protein and d-dimer, a marker of ongoing fibrin formation and degradation, were associated with poorer physical functioning in PAD patients. Measurements of walking distance, walking speed, and balance were significantly worse in patients with elevated C-reactive protein and d-dimer.
Infection
Although controversial, there is evidence that atherosclerosis may be associated with an inflammatory process caused by chronic infection with Chlamydia pneumoniae.88,89 Many existing studies have explored the role of Chlamydia species infection in coronary artery disease.90–92 A recent metaanalysis was performed in which the pooled data from 38 studies were examined.93 The overall odds ratio was 1.6, suggesting only a mild causative role at best. Skeptics argue that C. pneumoniae is an innocent bystander in the atherosclerotic process rather than a cause. In support of this argument, the association between atherosclerosis and C. pneumoniae infection appears to be higher in retrospective cross-sectional and case-control studies than in prospective case-control studies, and the association is inversely proportional to length of follow-up.93
Alcohol Consumption
Mild to moderate alcohol consumption is associated with a reduced risk of cardiovascular disease and reduced cardiac mortality.94–97 Several epidemiologic studies have suggested an inverse relationship between alcohol consumption and PAD.17,98–101 In nonsmoking men, researchers from the Rotterdam Study found an odds ratio for PAD of 0.68 with consumption of more than 20 g of alcohol per day, with an odds ratio of 0.41 in a comparable group of women. The beneficial effects of alcohol are thought to be due to its influence on hemostasis,81 its lipid profile,102 and the generation of oxygen free radicals.103
Natural History
Once the diagnosis has been made, patients and physicians fear both disease progression and limb loss, in addition to the functional limitations caused by intermittent claudication. Multiple longitudinal studies of large groups of claudicants with objective criteria for enrollment provide an accurate database.104–107
Vascular Overlap
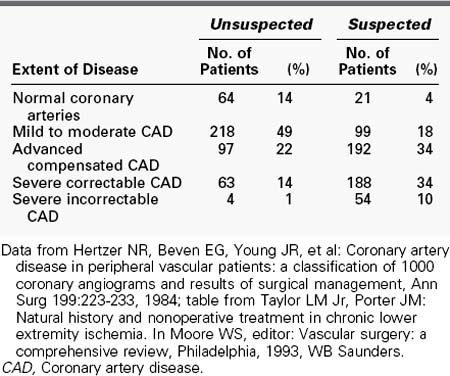
Because atherosclerosis is a systemic process, significant overlap exists between PAD and other forms of cardiovascular disease—namely, coronary artery disease and cerebrovascular disease. Hertzer and colleagues108 clearly demonstrated a high incidence of coronary artery disease in vascular surgery patients. Their conclusions were based on a series of 1000 patients undergoing major vascular surgery in whom they performed preoperative coronary angiography, regardless of the history of coronary artery disease or symptoms. More than 90% of patients had clinically significant coronary artery disease, much of which was asymptomatic. The authors also found an increased frequency of severe coronary artery disease with age, from 22% among patients younger than 50 years to 41% among patients 70 years or older. Table 32-3 summarizes the data from this important study.
TABLE 32-3 Incidence of Coronary Artery Disease in 1000 Consecutive Patients with Peripheral Vascular Disease Screened by Angiography
In the Cardiovascular Health Study, 60% of patients with PAD had a history of other symptomatic cardiovascular disease, such as myocardial infarction, angina, or stroke.12 Conversely, 40% of patients with coronary artery or significant cerebrovascular disease also had PAD. Similar findings were reported in the large epidemiologic study by Aronow and Ahn,8 in which 1886 patients older than 62 years were screened for cardiovascular disease. Seventy percent of patients with PAD had associated coronary artery or cerebrovascular disease (34% cerebrovascular, 58% coronary artery). The well-recognized overlap between PAD and other types of cardiovascular disease has been confirmed in numerous large epidemiologic studies and clinical trials (Table 32-4).
TABLE 32-4 Concomitant Cerebrovascular and Coronary Artery Disease in Patients with Peripheral Arterial Disease
| Study | Cerebrovascular Disease (%) | Coronary Artery Disease (%) |
|---|---|---|
| Ogren and colleagues (1993)257 | 33 | 51 |
| Szilagyi and colleagues (1986)258 | 19 | 47 |
| Mendelson and colleagues (1998)259 | 35 | 62 |
| Aronow and Ahn (1994)8 | 34 | 58 |
| CAPRIE (1996)123 | 19 | 40 |
| Meijer and colleagues (1998)10 | ||
| Men | 9 | 39 |
| Women | 8 | 14 |
CAPRIE, Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events.
Progression of Symptoms
Knowledge of the natural history of PAD is essential when planning therapeutic strategies. When patients with intermittent claudication are followed for 5 years, approximately 50% to 75% have either no change in symptoms or experience improvement. Approximately 25% experience symptom progression, with 5% to 25% requiring therapeutic intervention and only 2% to 4% requiring major amputation.19,109 Both continued tobacco use33 and diabetes mellitus109 are correlated with progressive deterioration. However, the most important consistent predictor is the severity of objectively determined arterial occlusive disease at the first patient encounter.51
Life Expectancy
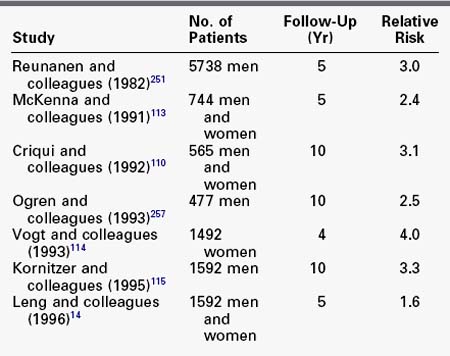
In contrast to the relatively benign lower extremity prognosis in most patients with PAD, the prognosis for morbidity and mortality from other manifestations of cardiovascular disease is worse. Life expectancy is clearly reduced in patients with PAD, attributable primarily to an increase in cardiovascular disease (Table 32-5).* The relative risk of a patient with intermittent claudication having a fatal or nonfatal myocardial infarction or stroke is twofold to threefold that of a nonclaudicant. All-cause mortality is also twofold to fourfold higher, with 60% of deaths from myocardial infarction and 15% from stroke.110,112 According to the TransAtlantic Inter-Society Consensus document, the 5-year mortality of the average claudicant is 30%, with the majority of deaths caused by manifestations of cardiovascular disease.20 An additional 5% to 10% will experience a nonfatal cardiovascular event.19 The prevalence of asymptomatic carotid artery disease in patients with chronic lower extremity disease has also been examined. Screening carotid duplex scans in patients requiring infrainguinal revascularization have demonstrated a 30% incidence of asymptomatic internal carotid artery stenosis greater than 50% in that population.116,117 Given the demonstrated prevalence of concomitant coronary and carotid vascular disease, reduced long-term survival in patients with chronic lower extremity ischemia is not surprising.
TABLE 32-5 Relative Risk of Mortality in Patients with Peripheral Arterial Disease Compared with Those Without
Multiple risk factors have been defined as important contributors to the increased long-term cardiovascular mortality of patients with lower extremity arterial occlusive disease. These include advanced age, continued tobacco use,34 diabetes,118 and dialysis dependence.119–121 Of these, end-stage renal disease is the most pronounced, predicting 2-year survival rates of 50% to 65%.
Survival is inversely related to the degree of objectively determined chronic lower extremity ischemia at presentation.122 McDermott and coworkers122 showed that patients with an ABI less than 0.3 had significantly higher mortality than those with an ABI of 0.3 to 0.9 (relative risk 1.8).122 In a group of patients followed for 10 years, Criqui and associates110 demonstrated progressively decreasing survival with increasing PAD disease severity. Among patients with a normal ABI, asymptomatic PAD, moderately symptomatic PAD, and severe symptomatic PAD, 10-year survival rates were approximately 85%, 55%, 40%, and 25%, respectively. In another study, Vogt and coworkers114 found that patients with multilevel PAD had a relative mortality risk of 7.2 compared with controls. In contrast, patients with PAD confined to the aortoiliac arterial segment had a relative mortality risk of 2.0 compared with controls. Multiple randomized trials in large populations with coronary disease indicate that aggressive risk factor modification reliably reduces near-term cardiac mortality.123–129
Critical Limb Ischemia
At the far end of the spectrum of clinical severity are those patients with critical limb ischemia (CLI). CLI is defined as arterial blood flow that is inadequate to accommodate the metabolic needs of resting tissue. Clinically, CLI describes a group of patients with limb-threatening ischemia and includes patients with rest pain, ischemic ulcerations, and gangrene. Objective circulatory measurement of CLI populations provides the best stratification of prognosis. The likelihood of near-term limb loss is related to the severity of ischemia at the time of patient presentation and the presence of tissue loss. Data on the incidence and prevalence of CLI are less definitive than data for intermittent claudication. Using multiple different extrapolation methods, it is estimated that between 500,000 and 1 million new cases occur each year.130,131 This means that 1 new patient per year develops CLI for every 100 patients with intermittent claudication in the population, mostly in older patients. Few epidemiologic studies of CLI exist, compared with the myriad studies addressing the epidemiology of intermittent claudication. In a 7-year prospective study from the Lombardy region of northern Italy, CLI was estimated by three methods: conversion of intermittent claudication to CLI in prospectively followed patients, hospital admissions for CLI over a 3-month period, and rates of major limb amputations.130 Surprisingly similar results were obtained with each method, with the incidence of CLI ranging from 450 to 650 cases per 1 million population per year. A national survey of the Vascular Surgical Society of Great Britain and Ireland found a similar incidence of 400 patients per 1 million population per year.131
Patients with CLI and those with intermittent claudication share similar risk factors, but most patients with CLI do not have a clear history of intermittent claudication. Major risk factors for advanced limb ischemia include age, smoking, and diabetes. The incidence of major amputation rises markedly with age. A Danish national discharge survey reported that the incidence of major lower extremity amputations increased from 0.3 per 100,000 per year for patients younger than 40 years to 226 per 100,000 per year for those older than 80 years. Patients with diabetes who smoke require amputation earlier in life than do patients without diabetes who do not smoke.131
Not all CLI is truly critical. Clearly, patients with progressive gangrenous changes and constant ischemic pain have an unstable clinical situation requiring prompt therapy. However, abundant clinical experience indicates that patients who have CLI manifested by intermittent rest pain may experience noticeable improvement at times, presumably secondary to improved cardiac hemodynamics. Small ulcerations may heal with protective dressings alone. Several randomized pharmacologic trials have documented ulcer healing in up to 40% of CLI patients randomized to placebo,132,133 although in most of these trials, fewer than half the control patients were alive without a major amputation after 6 months.134,135
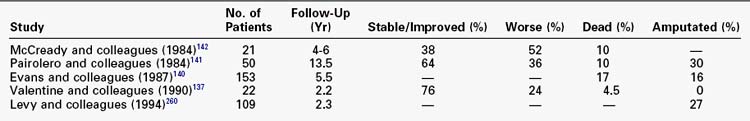
Chronic Lower Extremity Ischemia in Younger Patients
Lower extremity ischemia in patients younger than 40 years is infrequent. Peripheral vascular disease in young patients has several unique features that must be considered (Table 32-6).136–142 These patients are almost universally heavy smokers. One prospective study performed detailed evaluations for hypercoagulable states in younger patients with chronic lower extremity ischemia and demonstrated that 90% had laboratory abnormalities (deficiencies in natural anticoagulants, defective fibrinolytic activity, or the presence of antiphospholipid antibodies).139 Another study demonstrated significant abnormalities in LDL cholesterol oxidation in younger patients with PAD compared with older PAD patients.136
Despite attempts at revascularization, younger patients who manifest limb-threatening symptoms frequently progress rapidly to limb loss. Limited survival of reconstructions and the need for more frequent operative revisions characterizes these patients.136,143 Although survival is reduced in younger patients with peripheral vascular disease compared with age-matched controls, on balance, coronary atherosclerosis in younger patients with severe PAD does not appear to be as aggressive as the atherosclerosis affecting their lower extremities.144,145
Amputation
The incidence of major lower extremity amputation appears to have reached a plateau or decreased in the last decade, possibly owing to improved methods of revascularization and limb salvage, reduction in smoking, and improved diabetic control.146–148 As with the larger subgroup of patients with CLI, patients who undergo major amputation often have not experienced a steady disease progression from claudication to rest pain to tissue necrosis to amputation.
In a review of 713 patients who were undergoing below-knee amputations for ischemia, more than half had experienced no ischemic symptoms as recently as 6 months before the amputation.149
Overall, the ratio of below-knee amputations to above-knee amputations is equal and has not significantly changed in several decades.144,150–152 However, the introduction of aggressive limb salvage teams has increased the rate of below-knee amputations at selected centers.153 Primary healing of below-knee amputations ranges from 30% to 90%.153,154 Revision to attempt below-knee salvage varies from 4% to 30%.155–157 Half of all below-knee amputees who fail to achieve primary healing ultimately require above-knee amputation.158–160
More below-knee than above-knee amputees achieve ambulation.151,161,162 Overall, however, only a small number of major amputees for ischemic disease achieve meaningful independent ambulation. Initial rehabilitation can take 9 months or longer. After 2 years, 30% of amputees who had been walking no longer use their prostheses.151 Advanced age and female gender bode poorly for ambulation.163 Fifteen percent of amputees require contralateral amputation, and another 20% to 30% die within 2 years.151,153,164
Nonoperative Treatment
Management of Risk Factors
The first step in the management of PAD patients is treatment of risk factors. Multiple randomized trials in large populations at high risk for coronary disease indicate that aggressive risk factor modification (lipid reduction, antiplatelet therapy, diabetes management, and blood pressure control) reliably reduces near-term cardiac mortality.123,124,126–129 Table 32-7 describes basic guidelines for risk factor modification based on these trials.
TABLE 32-7 Recommendations for Risk Factor Reduction in Patients with Chronic Lower Extremity Ischemia
| Parameter | Target Goal | Therapy |
|---|---|---|
| LDL cholesterol | <100 mg/dL | Diet, statins |
| HDL cholesterol | Men, ≥35 mg/dL Women, ≥45 mg/dL fibrates | Diet, exercise, niacin |
| Triglycerides | <150 mg/dL | Diet, exercise, gemfibrozil, niacin |
| Blood pressure | Systolic <130 | β-Blockers |
| Diastolic | <85 | ACE inhibitors |
| Antiplatelet therapy | All patients on some form | Aspirin, clopidogrel |
| Diabetes | HbA1c < 7% | Insulin, ↑insulin sensitivity |
| Tobacco cessation | Complete abstinence | Nicotine replacement, antidepressant |
LDL, Low-density lipoprotein; HDL, high-density lipoprotein; ACE, angiotensin-converting enzyme; Hb A1c, glycosylated hemoglobin.
Smoking Cessation
Smoking cessation is by far the most important treatment for patients with PAD. PAD symptoms are unlikely to progress and may even improve once smoking is stopped completely.165 In patients with intermittent claudication, improvement in walking distance up to 40% has been reported.165,166 Improved patency of arterial repairs in nonsmokers has been demonstrated for both aortofemoral and femoropopliteal reconstructions,167–170 and the degree of tobacco use (measured by carboxyhemoglobin levels) bears directly on the incidence of graft occlusion.171
Patients with PAD are often unaware of the strong association between smoking and lower extremity disease. In one study, only 37% of smokers with PAD recognized smoking as a risk factor.172 The initial effort, therefore, on the part of all physicians must be to educate patients about the relationship between tobacco use and PAD and to inform patients unequivocally that smoking is the most important factor responsible for their leg condition. Studies have shown that strong and repeated advice by physicians to quit smoking results in abstinence in more than one third of smokers.173
Physicians must be prepared to provide a plan to help the patient achieve the goal of smoking cessation. Reassuring the patient is extremely important. Multiple attempts at quitting are common. For most people who eventually quit, 2 to 5 years and an average of six abstinence-relapse cycles are required.174 Although half of all smokers make an attempt to discontinue tobacco use each year, as few as 3% to 5% remain abstinent at 1 year.175 A recent randomized control trial demonstrated that patients enrolled in an intensive smoking cessation program consisting of education, counseling, behavioral therapy, and advice regarding pharmacologic adjuncts were threefold more likely to abstain from smoking at 6 months compared with patients only receiving advice from their physician.176 Pharmacologic adjuncts for the treatment of smoking addiction may be helpful in facilitating cessation. Current adjuncts include nicotine replacement therapy, partial nicotine receptor agonists, and antidepressant therapy. Most nicotine replacement agents provide up to 30% of a smoker’s regular daily nicotine intake and reduce or prevent withdrawal symptoms. Nicotine gum is the oldest form of nicotine replacement and is currently available without a prescription. Drawbacks include the requirement of specific chewing techniques to maximize nicotine release and drug inactivation with pH changes if beverages are consumed during use. Nicotine transdermal patches (dose ranging from 7 to 21 mg/24 hours) are easier to use. Reviews of randomized, double-blinded nicotine replacement trials for smoking cessation therapy in younger patients (30 to 40 years old) document biochemically confirmed 6-month abstinence rates of 20% to 45% in the treatment groups, compared with 5% to 25% in the control groups, depending on the setting (with treatment initiation in a smoking cessation clinic superior to that in a primary care office).177,178 No benefit was derived from treatment longer than 8 weeks or from the tapering of nicotine. Intermediate-dose (14 mg/24 hour) nicotine patches have been used cautiously in patients with symptomatic coronary artery disease.179
Partial nicotine receptor agonists, such as varenicline, have recently gained popularity as adjuncts to smoking cessation. A recent Cochrane database metaanalysis showed that varencicline appears to be superior to placebo and bupoprion for smoking cessation, but not significantly better than nicotine replacement therapy.180 Finally, nicotine and citrate inhalers have been used in several small, randomized trials with or without nicotine patches.181,182 These devices maintain reinforcement of the ritual and sensory phenomena of smoking. Although short-term abstinence with these devices has been achieved in 20% to 30% of cases, long-term success has been disappointing.
Smoking cessation programs now focus on depression as an important component of the smoker profile and as a major factor in withdrawal symptoms.183 The antidepressant agents buproprion and fluoxetine have been used in randomized trials, with 12-week biochemically confirmed cessation rates of 30% to 40%.184 In addition to diminishing withdrawal symptoms (which share many characteristics with chronic depression), these agents appear to attenuate some of the weight gain observed with smoking cessation. These agents have also been used in combination with nicotine replacement (patch or inhaler), with improved results compared with either agent alone.185
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree