Myocarditis
Michael H. Crawford, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() New congestive heart failure with a history of an antecedent viral syndrome.
New congestive heart failure with a history of an antecedent viral syndrome.
![]() Elevated cardiac biomarkers.
Elevated cardiac biomarkers.
![]() Electrocardiogram shows sinus tachycardia, nonspecific ST-T changes, atrial or ventricular arrhythmias, or conduction abnormalities.
Electrocardiogram shows sinus tachycardia, nonspecific ST-T changes, atrial or ventricular arrhythmias, or conduction abnormalities.
![]() Echocardiogram demonstrates chamber enlargement, wall motion abnormalities, systolic or diastolic dysfunction, or mural thrombi.
Echocardiogram demonstrates chamber enlargement, wall motion abnormalities, systolic or diastolic dysfunction, or mural thrombi.
![]() Endomyocardial biopsy reveals an inflammatory infiltrate with adjacent myocyte injury.
Endomyocardial biopsy reveals an inflammatory infiltrate with adjacent myocyte injury.
 General Considerations
General Considerations
Myocarditis is defined simply as an inflammatory process with necrosis that involves the myocardium. In the past, the myocardial injury was believed to be a direct result of the cytotoxic effects of the relevant organisms. Even as early as 1806, however, it was thought that a persistent inflammatory process following such an infection (eg, diphtheria) of the myocardium led to progressive cardiac damage and dysfunction. When the term “myocarditis” was first introduced in 1837 as inflammation or degeneration of the heart, the diagnosis could be made only postmortem. Fortunately, endomyocardial biopsy now allows the sampling of human myocardial tissue during life and thus the accurate antemortem diagnosis of myocarditis.
 Pathophysiology
Pathophysiology
The histologic hallmark of myocarditis is a focal patchy or diffuse inflammatory infiltrate with adjacent myocyte injury. The inflammation may not be restricted to the myocardium but may also involve the adjacent endocardium, pericardium, and valvular structures.
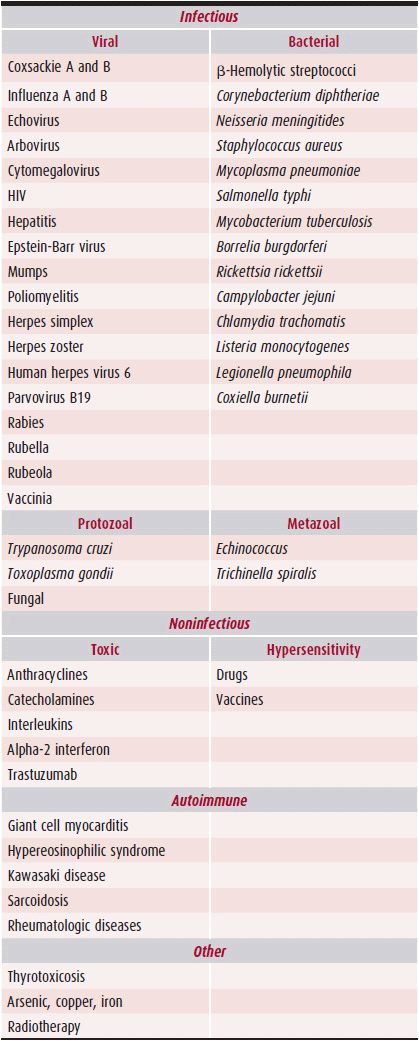
Myocarditis is most commonly initiated by viral infection (Table 25–1). Initiation of the pathophysiologic abnormalities, however, may result from a variety of insults, including drugs, toxins, hypersensitivity reactions, collagen vascular diseases, and autoimmune reactions. The most common viruses associated with myocarditis in the United States and Western Europe in immunocompetent persons are adenoviruses, coxsackievirus B (enterovirus), parvovirus B19, herpes simplex, influenza A, and cytomegalovirus (CMV). Other viruses, bacteria, rickettsiae, spirochetes, fungi, protozoans, or metazoans can also produce myocarditis; such causes are uncommon, however (see Table 25–1). Successful identification of the most common offending pathogens depends on knowledge of the geographic region’s relevant endemic and epidemic infectious diseases, the person’s immunization status and immunocompetence, and the sophistication and availability of public health services.
Table 25–1. Important Causes of Myocarditis
Several mechanisms of myocardial damage have been proposed. (1) Direct injury of myocytes by the infectious agent. (2) Myocyte injury caused by a toxin such as that from Corynebacterium diphtheriae. (3) Myocyte injury as a result of infection-induced immune reaction or autoimmunity.
The autoimmunity hypothesis is the most widely accepted theory. It is believed that the viral infection triggers a cell-mediated immunologic response that ultimately causes myocardial injury; the myocardial injury persists despite viral clearance.
In the murine model, coxsackievirus B3 causes an infectious phase, which lasts 7–10 days, and is characterized by active viral replication. During this phase, initial myocyte injury takes place, causing the release of antigenic intracellular components (such as myosin) into the bloodstream. Subsequently, after viral clearance, a second phase of myocyte damage will start. This phase is immune-mediated by CD8 lymphocytes and autoantibodies against various myocyte components. Antimyosin antibodies were isolated from mice that developed myocarditis following coxsackievirus B infection, as well as from patients with myocarditis. Antigenic mimicry, the cross reactivity of antibodies to both virus and myocardial proteins, occurs when an infectious agent shares an identical antigen with the normal myocyte. This mechanism is documented in animal models, and it may play a role in humans. Myocyte injury may be a direct result of CD8 lymphocyte infiltration. The local release of cytokines, such as interleukin-1, interleukin-2, interleukin-6, tumor necrosis factor (TNF), and nitric oxide may play a role in determining the T-cell reaction and the subsequent degree of autoimmune perpetuation. These cytokines may also cause reversible depression of myocardial contractility without causing cell death.
The popularity of the autoimmune hypothesis deemphasizes the role of the virus. Animal studies show, however, that viral proliferation itself might cause myocarditis. Some studies demonstrate the persistence of viral genomic fragments in myocardial cells of patients with active myocarditis and in some patients with dilated cardiomyopathy. Although these fragments may not be infectious, viral RNA may still serve as a persistent antigen to drive the immunologic response.
Exposure to cardiotropic viruses, presumably followed by a viral infection of the myocardium, is common. Based on the detection of serum antibodies to cardiotropic viruses, approximately 70% of the adult population has had prior exposure. Nonetheless, resultant abnormalities in cardiac function or symptomatic heart failure are unusual. The host factors predisposing to these deleterious immune responses are as yet undefined. Immunocompromised patients, such as pregnant women and patients with acquired immunodeficiency syndrome (AIDS), are predisposed to myocarditis. The susceptibility to viral myocarditis may also be age-related or, based on familial occurrence, genetically predetermined.
Kindermann I, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–92. [PMID: 22362396]
Mahrholdt H, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(5):1581–90. [PMID: 17015795]
 Clinical Findings
Clinical Findings
A. Symptoms & Signs
Myocarditis is most commonly asymptomatic, with no evidence of left ventricular dysfunction. The clinical manifestations of myocarditis are protean, when they are present. Myocardial involvement may be overshadowed or completely masked by the constitutional symptoms of the illness or other organ dysfunction. Cardiac symptoms may result from systolic or diastolic left ventricular dysfunction or from tachyarrhythmias or bradyarrhythmias. Patients frequently seek medical attention days to weeks after an acute febrile illness, particularly a flu-like syndrome. Common constitutional symptoms include fever, malaise, fatigue, arthralgias, myalgias, and skin rash.
Chest discomfort is a common symptom and is typically pericardial in nature; ischemic or atypical pain may also occur. Occasionally, patients have the syndrome of acute myocardial infarction with ischemic chest pain, electrocardiographic (ECG) abnormalities, elevated cardiac isoenzymes, or evidence of left ventricular wall motion abnormalities. Viral coronary arteritis and vasospasm have been implicated as the cause of this syndrome, but the epicardial coronary arteries are usually widely patent.
The acute onset of symptoms of congestive heart failure in a young person or in a patient without known coronary artery disease often suggests the diagnosis of myocarditis. Classic symptoms of congestive heart failure, including dyspnea, fatigue, decreased exercise tolerance, palpitations, and right heart failure, may be present. This constellation of signs and symptoms may be indistinguishable from dilated cardiomyopathy. It should be noted that because the metabolic demands on the heart associated with fever or a viral illness may initiate the first episode of congestive heart failure in patients with asymptomatic left ventricular dysfunction or reduced cardiac reserve, heart failure following a viral syndrome does not necessarily imply myocarditis.
Patients may also present with other symptoms that have been described in myocarditis: dizziness, syncope, or palpitations caused by atrial and ventricular arrhythmias and conduction disturbances. Myocarditis may present as sudden death, as a result of malignant ventricular arrhythmias or complete heart block; systemic and pulmonary thromboemboli have also been noted.
B. Physical Examination
The findings on physical examination vary widely. The patient may appear ill because the other manifestations of a viral illness dominate the clinical picture, and myocardial involvement may become evident only later in the course of the illness. Preexisting heart disease can also obscure the findings of myocarditis on examination.
Tachycardia, hypotension, and fever are associated with myocarditis. The tachycardia may be disproportionate to the degree of fever, and the heart rate is frequently elevated both at rest and with effort. Bradycardia is seen rarely, and a narrow pulse pressure is occasionally detected. Murmurs of mitral or tricuspid regurgitation are common, but diastolic murmurs are rare. The intensity of S1 may be decreased and the intensity of pulmonic closure increased, and S3 and S4 gallops may also be heard.
In more severe cases, congestive heart failure with distended neck veins, pulmonary rales, wheezes, gallops, and peripheral edema may be detected. Pleural and pericardial rubs are common in acute viral myocarditis, and a rhythm disturbance or conduction delay may be evident. Circulatory collapse and shock may occur, but these are rare.
C. Diagnostic Studies
1. Electrocardiography—ECG abnormalities are common in patients with myocarditis. These ECG changes are often nonspecific and transient, usually appearing only in the first 2 weeks of the illness. The most common abnormality is sinus tachycardia. While the presence of ST segment and T-wave changes suggests the diagnosis of myocarditis during a viral syndrome, subtle ECG changes may be caused solely by fever, hypoxia, hyperkalemia, and other metabolic abnormalities associated with the syndrome. Atrioventricular (AV) and intraventricular conduction delays are also common. Left bundle branch block occurs in approximately 20% of patients with active myocarditis. Complete AV block is not an uncommon finding and is often diagnosed after the patient presents with syncope. Heart block is usually transient but may occasionally require a temporary pacemaker. Supraventricular tachycardia is common, particularly with associated congestive heart failure or pericarditis. Ventricular ectopy may be the only clinical finding that suggests myocarditis. Other reported abnormalities include axis shifts and repolarization abnormalities.
2. Chest radiography—The chest radiograph may be normal, or it may demonstrate mild to moderate cardiomegaly from dilatation of the left or right ventricular cavity (or both). The cardiac silhouette may also be globular when a pericardial effusion is present, however. Evidence of venous congestion and pulmonary edema may be seen in more severe cases, and pulmonary infiltrates from concomitant pneumonia may be present.
3. Echocardiography—Echocardiography is a convenient and noninvasive method of evaluating chamber sizes, valvular function, and myocardial contractility. Left ventricular systolic dysfunction is commonly seen in patients with congestive heart failure. Regional wall motion abnormalities mimicking a myocardial infarction are surprisingly common; however, global hypokinesis may also occur. The left ventricular cavity may be normal in size or minimally enlarged; it may be markedly enlarged in those with fulminant disease. Mitral or tricuspid regurgitation may be present. Interestingly, an increase in wall thickness mimicking hypertrophic cardiomyopathy may be seen early in the course of the disease, presumably secondary to edematous inflammation. Mural thrombi occur in approximately 15% of cases. Echocardiography is also helpful in demonstrating abnormalities of diastolic filling that mimic restrictive cardiomyopathy and in distinguishing ventricular dilatation from pericardial effusion. Echocardiograms are commonly obtained serially to monitor the course of the illness and to evaluate therapy. Echocardiographic changes may persist, improve, or even worsen after clinical resolution of acute myocarditis.
4. Magnetic resonance imaging—Acute myocarditis can be detected by gadolinium-enhanced cardiovascular magnetic resonance imaging (MRI) using three primary imaging techniques. T2-weighted images readily detect myocardial edema, which is characteristic of inflammation. Early gadolinium enhancement in T1-weighted images identifies an increase in tissue blood volume due to vasodilatation from inflammation. Late gadolinium enhancement reflects myocardial fibrosis or necrosis, which is typical of the later stages of myocarditis. The best diagnostic accuracy compared to myocardial biopsy is when multiple criteria are used. If two of these three MRI abnormalities are found, the sensitivity in pooled studies is about 90%, specificity about 70%, and accuracy about 80%. Finding decreased left ventricular function, increased left ventricular cavity size, and increased myocar-dial volume are also supportive findings. MRI may help localize the areas where biopsy specimens should be taken. MRI is most often positive when biopsy specimens show definite myocarditis. Borderline biopsy criteria cases are less likely to be detected by MRI, and no MRI parameter predicts the presence of viral genomics by polymerase chain reaction (PCR).
5. Cardiac catheterization—Cardiac catheterization is not routinely performed in all cases of myocarditis; however, it may help in the diagnosis when the presentation mimics myocardial infarction. Characteristic hemodynamic findings of myocarditis include an elevated left ventricular enddiastolic pressure, a depressed cardiac output, and increased ventricular volumes. Ventriculography may also confirm abnormalities seen on echocardiography or MRI. Coronary angiogram typically demonstrates normal coronary arteries.
6. Endomyocardial biopsy—Myocardial biopsy is considered the gold standard for the diagnosis of myocarditis. It is an invasive procedure, although it only involves minimal morbidity and discomfort. The Stanford-Caves reusable bioptome is typically introduced to the right ventricular cavity. Newer, single-use bioptomes and sheaths can be introduced through right and left jugular, subclavian, or femoral veins and femoral arteries. Four to six tissue fragments are obtained, usually from the right side of the intraventricular septum, but cardiovascular MRI can be used to direct the bioptome to areas of focal disease. The major risk of myocardial biopsy is cardiac puncture. It occurs in less than 1% of cases but is often fatal. Computed tomography or three-dimensional echocardiography guidance can reduce the risk of right ventricular free wall puncture.
The histologic criteria for the diagnosis of myocarditis are defined as an inflammatory infiltrate of the myocardium with injury to the adjacent myocytes not typical for the ischemic damage associated with coronary artery disease. The diagnosis of borderline myocarditis is made when the infiltrate is not accompanied by myocyte injury. In large trials, only about 10% of patients with unexplained congestive heart failure met the criteria for active myocarditis. Many patients with negative biopsies have the classic clinical presentation of myocarditis. One explanation for the low yield of endomyocardial biopsy is that the inflammation may be focal or patchy. Another reason might be that some patients have purely humoral or cytokine-mediated forms of myocarditis with little or no cellular infiltrate. Furthermore, the histologic changes may be transient with rapid resolution. It should be noted that endomyocardial biopsy may rule in, but never rule out active myocarditis.
In recent years, the role of endomyocardial biopsy has changed. It is no longer mandatory and essential in the evaluation of unexplained heart failure because the information it provides will rarely determine specific therapy. This became particularly evident after publication of the results of the Myocarditis Treatment Trial, which failed to show any benefit of the immunosuppressive therapy. It remains important to consider biopsy in some special cases of myocarditis, particularly in patients who do not respond to conventional therapy. Some patients in this group may have giant cell myocarditis, which requires a more aggressive approach with immunosuppressive therapy and consideration for early transplantation. Current guidelines suggest that biopsy may be appropriate in cases of unexplained new-onset (< 2 weeks) heart failure. After 2 weeks, no response to treatment or suspicion of special diagnoses, such as eosinophilic myocarditis, are considered valid indications for biopsy.
7. Other tests—An elevated erythrocyte sedimentation rate (ESR) is detected in approximately 60% of patients with active myocarditis. If the ESR is elevated, it may help monitor the course of the illness and effectiveness of therapy. The accuracy of this test may be affected by coexisting hepatic congestion or hepatitis; these conditions decrease the synthesis of fibrinogen and lower the ESR.
Mild to moderate leukocytosis occurs in approximately 25% of patients, along with neutrophilia or lymphocytosis and occasionally eosinophilia, particularly in parasitic illnesses. The percentage of eosinophils may also increase in the recovery phase of myocarditis.
The creatine phosphokinase-myocardial band (CK-MB) fraction is elevated in approximately 6% of patients, with the degree of elevation being proportional to the degree of myocyte injury. Cardiac troponin-I (Tn I) is a sensitive and specific marker for myocyte injury and is increased in about one-third of patients with myocarditis.
Measurement of serum antibody titers to various cardiotropic viruses is useful for establishing exposure to these agents. The titers may be neutralizing antibodies, complement-fixing antibodies, or hemagglutination-inhibiting antibodies. Because a fourfold rise in titer over a 4–6 week period is required to document an acute infection, serial blood samples must be obtained. It must be kept in mind that an elevated antibody titer or rise in dilution only implies infection with the offending organism. Proof of active myocarditis also requires a positive biopsy result. Cultures of throat washings, urine, and feces may help identify a viral pathogen. Unfortunately, viral cultures are usually negative, and serologic studies are often nondiagnostic. In addition, viral recovery is usually possible only during the acute phase of the illness when active replication is occurring. Because this phase is not associated with viral injury, the diagnostic yield of culture of myocardial samples obtained by endomyocar-dial biopsy is minimal. Other laboratory analyses that may be useful include a monospot test, Epstein-Barr virus titers, hepatitis serology, and urine and serum for CMV.
The detection of viral genomic material in endomyocardial biopsies using recombinant DNA techniques is a promising diagnostic tool. Two methods are used: PCR and in situ hybridization. Plus-strand RNA indicates persistent viral state, and minus-strand RNA indicates active viral replication. Unfortunately, studies have been inconsistent, with viral detection ranging between 10% and 60% of patients with dilated cardiomyopathy and myocarditis, compared with almost no viral detection in the control groups. Some studies have noted a relationship between viral persistence and progressive myocardial dysfunction. At the present time, routine viral study of endomyocardial biopsy is not recommended and remains investigational with the possibility of clinical application in the future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


