Chapter 17 Myocardial Perfusion
Magnetic Resonance Imaging
INTRODUCTION
The presence of a hemodynamically significant coronary stenosis can be detected by studying its influence on the downstream microcirculation. As the stenosis worsens, myocardial perfusion is preserved via autoregulatory mechanisms that result in dilation of the arteriolar bed.1 When challenged with vasodilators, blood flow will not increase in myocardial segments supplied by a significantly stenosed coronary artery because the perfusion reserve has already been exhausted by autoregulation, whereas perfusion will increase substantially in territories supplied by normal coronary arteries. Myocardial perfusion imaging can therefore identify the presence of a flow-limiting coronary stenosis by detecting regional variations in perfusion reserve.
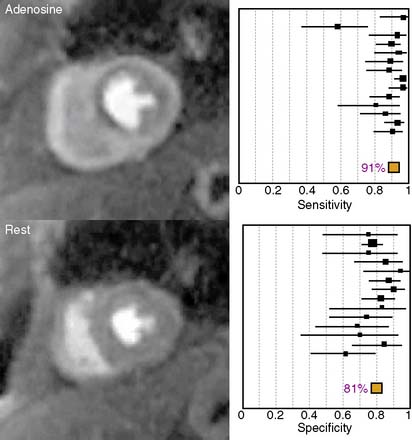
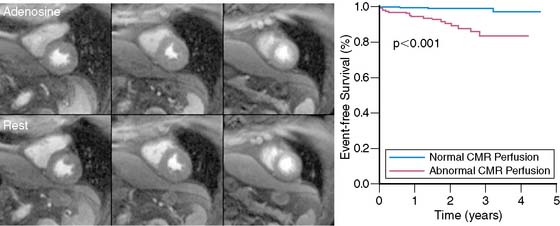
The diagnostic performance of perfusion CMR was recently evaluated in a meta-analysis of 14 studies including a total of 1183 patients.2 When compared to x-ray angiography, the sensitivity and specificity for detecting significant coronary artery disease were 91% and 81%, respectively (Fig. 17-1). In addition to its ability to identify patients with flow-limiting coronary disease, perfusion CMR readily differentiates low-risk from high-risk patients (Fig. 17-2). Three recent studies have prospectively studied the ability of perfusion CMR to identify low-risk patients. In these studies, only 4 of the 625 patients with no evidence of ischemia had a major adverse cardiac event after 1 year of follow-up.3–5 Stress testing in the MR environment is safe. Bernhardt et al. reported on the safety of performing an adenosine stress test in a MRI scanner.6 Of the 3174 patients studied, only one had a major complication (grand mal seizure). Minor side effects were experienced by 35% of the patients (e.g., chest pain, dyspnea, transient AV block, nausea). In this chapter, we will discuss the basic principle of perfusion CMR image acquisition and review various approaches used for image analysis.
BASIC PRINCIPLE OF IMAGE PREPARATION AND ACQUISITION
Regardless of the imaging modality used for assessing myocardial perfusion, the signal measured must adequately differentiate normally perfused myocardial segments from abnormally perfused segments. Contrast-enhanced CMR was initially used to estimate the perfusion reserve by Miller et al. in 1989.7 Atkinson et al. developed a technique a year later in 19908 that enabled a more dynamic evaluation of perfusion using first-pass imaging. Images are serially acquired in one or more preselected imaging planes as an intravenously administered bolus of a gadolinium chelate transits the vasculature and eventually the myocardium. As gadolinium migrates through the right-sided cardiac chambers, the pulmonary vasculature, the left-sided chambers, and ultimately the myocardium, a bright signal is generated. The rate of signal increase as the contrast agent initially perfuses a tissue is a gauge of blood flow.9
The signal generated and how closely it correlates to myocardial blood flow is also a function of the specific CMR pulse sequence parameters used. In order to generate a signal using magnetic resonance, the magnetization must be manipulated by applying a brief radiofrequency pulse to flip the proton alignment into the transverse plane. Once the radiofrequency pulse is completed, the protons return to their previous alignment. The rate at which the proton alignment recovers determines the longitudinal relaxation time, T1, of the tissue. Gadolinium is a paramagnetic contrast agent that shortens the T1 of neighboring protons, ultimately causing increased signal intensity in a concentration-dependent manner for T1-weighted images. In addition to the gadolinium concentration, the magnitude of the T1-weighted signal is dependent on the type of magnetization preparation used. The initial perfusion CMR studies were performed using an inversion recovery sequence.8 Because inversion recovery can be used to null the signal from tissues that are not exposed to gadolinium, a large signal contrast exists between hypoperfused and normally perfused tissues. However, magnetization preparation using inversion recovery is less robust in the presence of arrhythmias and is time- consuming, which compromises temporal resolution and limits the number of left ventricular slices that can be acquired during one cardiac cycle.
A more commonly used magnetization preparation scheme is saturation recovery.10 Saturation recovery is insensitive to arrhythmias, has a shorter acquisition window than inversion recovery, and more readily allows multislice coverage with an improved temporal resolution at the expense of reduced signal contrast between normally perfused and hypoperfused tissues. In experimental models and in clinical practice, the resultant contrast is sufficient to make a diagnosis.11 The amount of signal generated by a given concentration of gadolinium also depends on the time delay between magnetization preparation and image acquisition.12 Specifically, shorter delays allow for improved discrimination of higher concentrations of gadolinium, whereas longer delays allow for improved differentiation between lower concentrations of gadolinium.
Another important component of the CMR pulse sequence is the method of image acquisition. Currently, three approaches are most commonly used: gradient recalled echo (GRE),8 hybrid GRE and echoplanar imaging (GRE-EPI),13 and balanced steady-state free precession (SSFP).14 Each approach has its unique set of advantages and disadvantages. No consensus exists as to the best technique for image acquisition. The use of parallel imaging shortens the time necessary to acquire an image and likely reduces blurring and improves image quality of all three approaches.15,16
Of the three techniques, SSFP-based approaches have the highest contrast-to-noise ratio, and the signal is most linearly related to the underlying gadolinium concentration.17 However, the technique also requires a longer imaging duration, resulting in more blurring and motion artifact; SSFP also suffers most from susceptibility artifact. GRE-EPI has the most extensive spatial coverage and the shortest imaging duration, which results in less blurring and better image quality at higher heart rates. The signal is also linearly correlated with the concentration of gadolinium, but higher gadolinium concentrations are underestimated, and the overall CNR is less than that for SSFP-based images. GRE-based techniques have the lowest contrast-to-noise ratio and the poorest relationship between signal and gadolinium concentration. Its image duration is similar to SSFP, but the image quality may be better than SSFP at higher heart rates.
An important challenge of perfusion CMR is the so-called “dark rim artifact,” which mimics a hypoperfused segment on the subendocardial border of the myocardium.18,19 The etiology of the artifact is not clearly defined but appears to be related to cardiac motion, partial volume effects between the left ventricular cavity and the myocardium, and sudden changes in the concentration of contrast agent in the left ventricular blood pool and the myocardium. Whatever the etiology, the artifact is less pronounced when the hybrid GRE-EPI pulse sequence and parallel imaging are used.
APPROACHES TO IMAGE ANALYSIS
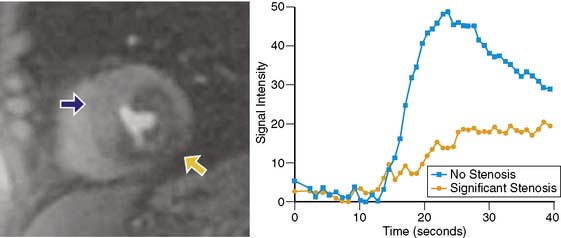
Because of its dynamic nature and versatility, perfusion CMR images can be analyzed qualitatively or quantitatively using time-intensity curves generated as the contrast agent perfuses the myocardium. Because qualitative interpretation can be performed immediately after image acquisition and does not require extensive postprocessing, it has been the most accepted in clinical practice. Atkinson et al. initially used CMR to visually detect first pass of gadolinium in humans in 1990.8 Since then, significant improvements in hardware and pulse sequence development allow for multislice imaging with high temporal resolution. Typically, complete images for at least three left ventricular short-axis slices are acquired during each cardiac cycle. As the contrast agent traverses the cardiac chambers and the myocardium, serial images are acquired for approximately 50 beats. Myocardial segments that are supplied by a severe coronary stenosis have a slower rise in signal intensity and become hypointense compared to normal segments (Fig. 17-3).9 The hypointensity is typically present in a coronary distribution and is most pronounced in the subendocardium and can extend into the midmyocardium.
Although the visual interpretation of these abnormal perfusion patterns is clearly subjective, inter-reader agreement is quite good.20 Because CMR is not limited by attenuation artifacts and has high spatial resolution, perfusion CMR may have an important clinical role for the detection of significant coronary artery disease. The ability of CMR to detect perfusion defects without being hindered by attenuation artifact was initially exploited by McCrohon et al.21 They studied 30 patients with a presumed inferior-wall attenuation artifact on single-photon emission computed tomography (SPECT) imaging. CMR confirmed the absence of a perfusion defect in 77% of patients and detected ischemia in the remaining 23%. In 104 patients with no history of prior myocardial infarction, Ishida et al. reported the diagnostic performance of perfusion CMR for detecting coronary stenoses at greater than 70%.22 In this patient population, the sensitivity was 90% and the specificity was 85%. As anticipated, the sensitivity was higher in patients with three-vessel coronary artery disease and lowest for detecting single-vessel disease. A subgroup of 69 patients also had SPECT imaging. Based on receiver operating characteristic (ROC) analysis, perfusion imaging using CMR performed better than nongated SPECT without attenuation correction. On the other hand, Sakuma et al. also compared x-ray angiography from 40 patients with both CMR and SPECT and found that there was no statistically significant difference in ROC for the two diagnostic tests.23
Although several small studies have evaluated the accuracy of perfusion CMR, it is not clear what the optimal contrast dose is for visual image interpretation. A recent multicenter, multivendor, prospectively designed trial of 234 patients tested five different doses of gadolinium ranging from 0.01 mmol/kg to 0.1 mmol/kg, and concluded that 0.1 mmol/kg was optimal.24 In the subgroup of patients who received this higher dose of gadolinium (n = 42), perfusion CMR performed at least as well as SPECT for detecting significant coronary disease. Based on ROC analysis, the area under the curve was 0.86 ± 0.06 (sensitivity 85%, specificity 67%) for CMR and 0.75 ± 0.09 for nongated SPECT (P = 0.12). In a total of 99 patients, another multicenter trial20 also evaluated the influence of three different doses of gadolinium (0.05 mmol/kg, 0.1 mmol/kg, and 0.15 mmol/kg) on the diagnostic performance of adenosine perfusion CMR. In this trial, the lower contrast agent dose outperformed the two higher doses. The sensitivity for detecting coronary stenoses greater than 70% was 93%, specificity was 75%, and accuracy was 85%. Although the preliminary data evaluating whether visual interpretation of perfusion CMR images are promising, the optimal contrast dose is not clearly defined, and it is unknown whether it will perform significantly better than gated SPECT utilizing attenuation correction. Large prospectively designed multicenter trials comparing the two modalities are still needed.
In addition to comparisons to SPECT imaging, first-pass vasodilator perfusion CMR has been compared to dobutamine functional CMR.25 Paetsch et al. studied 79 patients scheduled for x-ray angiography with both dobutamine and adenosine stress CMR. Adenosine perfusion imaging had a higher sensitivity (91% versus 89%) and a lower specificity (62% versus 80%), but the differences were not statistically significant. However, the development of an adenosine-induced wall motion abnormality was highly specific for the presence of significant coronary artery disease and occurred only in the presence of nearly transmural perfusion defects.
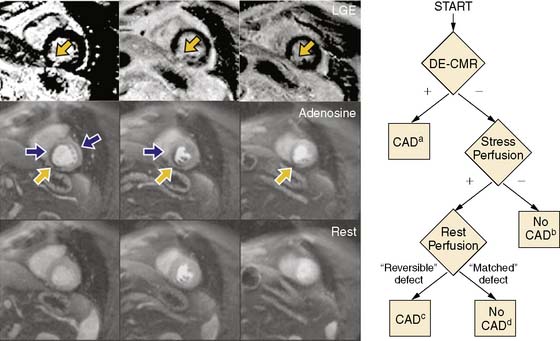
Although advantages of CMR perfusion imaging include its high spatial and temporal resolution, an important limitation is its ability to differentiate the problematic dark rim artifact from ischemia.19 Recently, Klem et al. proposed an algorithm utilizing late gadolinium enhancement scar imaging to help differentiate true perfusion defects from artifacts.26 In the algorithm, late gadolinium enhancement images are evaluated for the presence of a scar in a coronary distribution. Those patients with a subendocardial scar are diagnosed with significant coronary artery disease. If no scar is present, perfusion images acquired during hyperemia are analyzed for the presence of a perfusion defect. If no perfusion defect is present, significant obstructive coronary disease is excluded. If a perfusion defect is present, resting perfusion images are examined. All reversible perfusion defects are considered true ischemia, whereas perfusion abnormalities that persist on the resting images are attributed to artifact, because segments with a scar have already been accounted for earlier in the algorithm (Fig. 17-4). When compared to perfusion imaging alone, the combination of perfusion and scar imaging had an improved sensitivity (89% versus 84%), specificity (87% versus 58%), and diagnostic accuracy (88% versus 68%) in the 92 patients studied. But another study that included patients with a prior history of myocardial infarction27 concluded that the additive value of late gadolinium imaging was not as apparent and may even reduce the specificity for detecting a significant stenosis (87% versus 80%).