We aimed to determine whether the myocardial extracellular volume (ECV), measured using T1 measurements obtained during cardiac magnetic resonance imaging were increased in patients treated with anthracyclines. We performed cardiac magnetic resonance imaging and echocardiography and measured the ECV in 42 patients treated with anthracyclines. The data from the cardiac magnetic resonance study were compared to those from healthy volunteers. The anthracycline-treated cohort consisted of 21 men and 21 women with a mean age of 55 ± 17 years, who presented a median of 84 months after chemotherapy with a cumulative anthracycline exposure of 282 ± 65 mg/m 2 and a mean left ventricular ejection fraction of 52 ± 12%. The ECV was elevated in the anthracycline-treated patients compared to the age- and gender-matched controls (0.36 ± 0.03 vs 0.28 ± 0.02, p <0.001). A positive association was found between the ECV and left atrial volume (ECV vs indexed left atrial volume, r = 0.65, p <0.001), and negative association was found between the ECV and diastolic function (E′ lateral, r = −0.64, p <0.001). In conclusion, the myocardial ECV is elevated in patients with previous anthracycline treatment and is associated with the diastolic function and increased atrial volumes.
The detection of anthracycline-induced myocardial injury is limited by the long latency period between the injury and clinical presentation, and the methods of assessment used. Furthermore, the measurements are usually within the normal range, missing the myocyte damage that occurs before a decline in the ejection fraction (EF), and leading to the incorrect assumption that injury is absent. Anthracycline therapy results in myocardial fibrosis (MF). Cardiac magnetic resonance (CMR) imaging with late gadolinium enhancement (LGE) is the reference standard for the noninvasive detection of MF. However, LGE is an infrequent finding in patients treated with anthracyclines, despite a reduced EF. In contrast, MF, as detected by pathologic examination, is ubiquitous in the hearts of patients treated with anthracyclines. Current CMR techniques require a normal reference myocardium, but anthracycline-cardiomyopathy is associated with diffuse myocardial involvement. To address this limitation, a CMR-based technique has been developed in which the measurement of myocardial T1 provides a quantitative measure of the myocardial extracellular volume (ECV) and reflects the pathologic extent of MF, and is associated with cardiovascular mortality. Therefore, the goal of the present study was to test whether the ECV is abnormal in patients treated with anthracyclines and to test the associations between the ECV and other indexes.
Methods
The institutional human subjects review committee of our institution approved the protocol. We performed an observational study of patients with previous anthracycline treatment who had been referred for a clinically indicated CMR study from 2010 to 2012. Healthy controls for the CMR measures were recruited by open invitation. The requirements for inclusion were a history of anthracycline treatment, sinus rhythm, and the absence of an alternate pathologic etiology for myocardial ECV expansion. Significant coronary artery disease was excluded by either negative coronary angiographic findings or negative stress testing findings with imaging. Heart failure was determined by a combination of the results of a detailed questionnaire at the CMR study, a measurement of cardiac function, and a review of the medical record for a history of heart failure. The results for the CMR indexes were compared to those for age- and gender-matched healthy volunteers (n = 15). The volunteers were free of hypertension, diabetes, known coronary disease, malignancy, or sleep apnea.
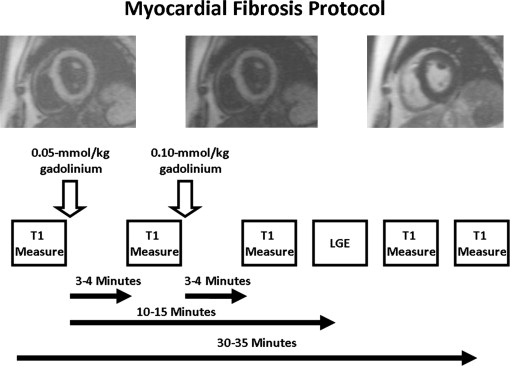
All images were acquired with electrocardiographic gating and breath-holding and with the patient in the supine position. The subjects underwent imaging with a 3.0-T CMR system (Tim Trio, Siemens, Erlangen, Germany), and measurements for left ventricular size, function, and mass, left atrial volume, T2-weighted sequences, and LGE were performed, as previously described. Gadopentetate dimeglumine at a dose of 0.15 mmol/kg (MAGNEVIST, Bayer HealthCare Pharmaceuticals, Leverkusen, Germany) was used as the contrast agent for all imaging studies in both healthy volunteers and patients. T1 measurements were performed using a Look-Locker sequence with a nonslice-selective adiabatic inversion pulse, followed by segmented gradient-echo cine acquisition for 17 cardiac phases/times after inversion, spread over 2 cardiac cycles (temporal resolution 80 to 100 ms before contrast and 45 to 55 ms after contrast, slice thickness 8 mm, repetition time >3 RR intervals before contrast and 2 RR intervals after contrast). The sequence was performed in a single, mid-left, ventricular slice before contrast and was repeated in the same mid-left ventricular short-axis slice 3 to 5 additional times after the injection of gadolinium ( Figure 1 ). The second T1 measurement was performed 3 to 4 minutes after the initial dose of contrast (0.05 mmol/kg). Immediately after this second T1 measurement, a second dose of contrast was given (0.10 mmol/kg), because LGE imaging was also performed for completeness. The third T1 measurement was performed 3 to 4 minutes after this second dose of contrast. The final T1 measurement was performed just before the end of the study, an average of 30 to 35 minutes after the initial dose of contrast. Either 1 or 2 other T1 measurements were performed after the third measurement and before the final measurement at no preset intervals. The signal intensity versus time curves for each segment and the blood pool were used to determine the segmental T1 through fitting to an analytical expression for the inversion recovery signal intensity. The reciprocal of T1 (R1 = 1/T1) was used to plot the myocardial R1 against the R1 in the blood pool and to calculate the slope by linear regression analysis, using all measurement points with a blood pool R1 of <3 s −1 . From the slope of the relation, the partition coefficient, the ECV, was obtained by multiplying each of the segmental partition coefficient by (1 − hematocrit in %/100), as reported previously. An ECV was then calculated by averaging the 6 myocardial segmental values from the mid-left ventricular short-axis slice. Segments containing LGE were excluded from the analysis.
The Vivid-7 ultrasound system (GE Healthcare, Milwaukee, Wisconsin) was used for standard and tissue Doppler echocardiography. Left ventricular measurements were performed as recommended by the American Society of Echocardiography. Tissue Doppler myocardial velocities were acquired in early diastole (E′) at the lateral and septal aspects of the mitral annulus in the apical 4-chamber view.
A comparison of continuous data between the combined groups was made using either an unpaired Student t test or a Wilcoxon rank-sum test. Nominal data were compared using Fisher’s exact test. We assessed the inter- and intraobserver variability in a randomly selected group of 16 subjects. A comparison of the interobserver and intraobserver characteristics of the myocardial ECV was made using Bland-Altman plots and determination of the 95% limits of agreement between methods. p Values <0.05 were deemed significant, and SAS (SAS Institute, Cary, North Carolina) was used for statistical analysis.
Results
We studied 42 patients with previous anthracycline-based chemotherapy ( Table 1 ). Of the 42 patients, 9 had Hodgkin’s lymphoma, 17 had non-Hodgkin’s lymphoma, 7 had breast cancer, 5 had leukemia, and 4 had bone cancer. Also, 2 patients had received liposomal doxorubicin and 2 trastuzumab. We separated the anthracycline-treated group according to whether the EF was preserved (n = 28) or reduced (n = 14). The preserved EF group was referred for a CMR study before ablation of atrial fibrillation (n = 14) or for workup of heart failure (n = 14). The group with a reduced EF were referred for a CMR study for workup of heart failure with a reduced EF (n = 14; Table 1 ). The echocardiographic parameters are listed in Table 2 . The tissue-Doppler derived E′ was lower in patients with previous anthracycline use and a reduced EF compared to those with a preserved EF.
| Variable | CTRL (n = 15) | AC-ALL (n = 42) | AC-PEF (n = 28) | AC-REF (n = 14) | p Value | |
|---|---|---|---|---|---|---|
| AC-ALL vs CTRL | AC-PEF vs AC-REF | |||||
| Age at chemotherapy (yrs) | — | 47 ± 17 | 48 ± 19 | 47 ± 16 | 0.78 | |
| Mean interval ∗ (mo) | — | 89 ± 40 | 83 ± 30 | 105 ± 49 | 0.99 | |
| Age at cardiac magnetic resonance study (yrs) | 56 ± 13 | 55 ± 17 | 56±17 | 56 ± 15 | 0.78 | 0.99 |
| Men | 7 (47%) | 21 (50%) | 13 (46%) | 8 (57%) | 0.81 | 0.74 |
| Anthracycline dose (mg/m 2 ) | — | 282 ± 65 | 272 ± 59 | 301 ± 72 | 0.17 | |
| Chest radiotherapy | — | 12 (29%) | 7 (25%) | 5 (36%) | 0.49 | |
| Risk factors | ||||||
| Hypertension | — | 14 (33%) | 10 (36%) | 4 (29%) | 0.74 | |
| History of atrial fibrillation | — | 19 (41%) | 15 (54%) | 4 (29%) | 0.19 | |
| Family history of cardiomyopathy | — | 5 (12%) | 3 (11%) | 2 (14%) | 1.00 | |
| Body mass index (kg/m 2 ) | — | 28 ± 4 | 27 ± 4 | 28 ± 5 | 0.98 | |
| Blood pressure (mm Hg) | ||||||
| Systolic | 124 ± 10 | 114 ± 16 | 114 ± 18 | 116 ± 16 | 0.03 | 0.73 |
| Diastolic | 75 ± 9 | 71 ± 12 | 71 ± 8 | 71 ± 13 | 0.18 | 0.95 |
| Heart rate (beats/min) | 72 ± 11 | 78 ± 13 | 78 ± 12 | 77 ± 14 | 0.001 | 0.90 |
| Medication | ||||||
| Angiotensin-converting enzyme/angiotensin receptor blocker | — | 33 (76%) | 20 (71%) | 13 (93%) | 0.23 | |
| β blocker | — | 37 (88%) | 24 (86%) | 13 (93%) | 0.65 | |
| Spironolactone | — | 3 (7%) | 0 (0%) | 3 (21%) | 0.03 | |
| Diuretics | — | 29 (69%) | 17 (61%) | 12 (86%) | 0.16 | |
| Antiarrythmics | — | 13 (31%) | 10 (36%) | 3 (21%) | 0.49 | |
| Warfarin | — | 14 (33%) | 12 (43%) | 2 (14%) | 0.09 | |
| Aspirin | — | 12 (29%) | 11 (39%) | 1 (7%) | 0.04 | |
| Statin | — | 16 (38%) | 11 (39%) | 5 (36%) | 1.00 | |
| Glomerular filtration rate † (ml/min/1.73 m 2 ) | — | 95 ± 31 | 99 ± 24 | 87 ± 28 | 0.21 | |
| Hematocrit | 42 ± 4 | 41 ± 3 | 41 ± 3 | 40 ± 3 | 0.56 | 0.94 |
∗ From chemotherapy to clinical presentation for heart failure or atrial fibrillation.
| Variable | AC-ALL (n = 42) | AC-PEF (n = 28) | AC-REF (n = 14) | p Value (AC-PEF vs AC-REF |
|---|---|---|---|---|
| Left ventricular internal dimensions in diastole (mm) | 50 ± 6 | 47 ± 4 | 55 ± 4 | <0.001 |
| Left ventricular ejection fraction (%) | 51 ± 12 | 58 ± 5 | 37 ± 7 | <0.001 |
| Pulmonary artery systolic pressure (mm Hg) | 36 ± 8 | 34 ± 7 | 39 ± 10 | 0.05 |
| Transmitral E (cm/s) | 75 ± 20 | 74 ± 16 | 75 ± 22 | 0.97 |
| Transmitral A (cm/s) | 73 ± 16 | 72 ± 14 | 76 ± 18 | 0.45 |
| E/A | 1 ± 0.4 | 1.1 ± 0.4 | 1 ± 0.3 | 0.51 |
| E′ medial (cm/s) | 6.2 ± 1.1 | 6.5 ± 0.8 | 5.4 ± 0.7 | <0.001 |
| E′ lateral (cm/s) | 6.6 ± 1.5 | 7.1 ± 0.9 | 5.9 ± 1 | 0.01 |
| E/E′ | 12 ± 4 | 12 ± 3 | 13 ± 4 | 0.15 |
MF by LGE imaging was an infrequent finding, observed in 3 patients ( Table 3 ). It was found in a basal distribution. No quantitative or qualitative evidence was found of edema in the anthracycline-treated patients on T2-weighted imaging (relative T2 signal intensity 1.6 ± 0.2). The mean differences within and between observers for the ECV measurement were acceptable ( Figure 2 ). The average ECV in healthy controls was 0.28 ± 0.02. The myocardial ECV was elevated in patients treated with anthracycline-based chemotherapy compared to healthy controls ( Table 3 ). No segmental variation in the ECV was found in the patients (0.35 ± 0.03, 0.36 ± 0.03, 0.35 ± 0.03, 0.37 ± 0.03, 0.37 ± 0.03, and 0.36 ± 0.03, clockwise from anterior to the anteroseptal segment, p = 0.57). No association was seen between the ECV and the heart rate (r = −0.06) or blood pool T1 values (r = −0.08). A direct association was found between the ECV and the indexed left atrial volume ( Figure 3 ), with an inverse association between the ECV and both the medial E′ ( Figure 3 ) and the lateral E′ ( Figure 3 ) and a direct association between the ECV and the ratio of the transmitral E wave to the tissue Doppler-derived E′ ( Figure 3 ). The ECV was greater in patients with a reduced EF than in those with a preserved EF ( Figure 4 ).
| Variable | CTRL (n = 15) | AC-ALL (n = 42) | AC-PEF (n = 28) | AC-REF (n = 14) | p Value | |
|---|---|---|---|---|---|---|
| AC-ALL vs CTRL | AC-PEF vs AC-REF | |||||
| Left ventricular diastolic volume (ml) | 133 ± 25 | 175 ± 43 | 161 ± 34 | 201 ± 45 | 0.001 | 0.003 |
| Left ventricular diastolic volume index (ml/m 2 ) | 71 ± 12 | 90 ± 21 | 84 ± 17 | 101 ± 22 | 0.002 | 0.01 |
| Left ventricular systolic volume (ml) | 51 ± 12 | 86 ± 38 | 67 ± 16 | 125 ± 45 | 0.001 | <0.001 |
| Left ventricular systolic volume index (ml/m 2 ) | 27 ± 7 | 44 ± 19 | 35 ± 8 | 63 ± 21 | 0.002 | <0.001 |
| Left ventricular ejection fraction (%) | 62 ± 5 | 52 ± 12 | 58 ± 8 | 38 ± 8 | 0.004 | <0.001 |
| Left ventricular mass (g) | 117 ± 13 | 106 ± 28 | 111 ± 17 | 96 ± 10 | <0.001 | <0.001 |
| Left ventricular mass index (g/m 2 ) | 65 ± 6 | 55 ± 16 | 58 ± 16 | 48 ± 7 | 0.002 | <0.001 |
| Maximum left atrial volume (ml) | 66 ± 14 | 113 ± 29 | 102 ± 18 | 136 ± 2 | <0.001 | <0.001 |
| Indexed maximum left atrial volume (ml/m 2 ) | 37 ± 9 | 58 ± 14 | 47 ± 10 | 68 ± 12 | <0.001 | <0.001 |
| Right ventricular diastolic volume (ml) | 128 ± 26 | 142 ± 37 | 132 ± 33 | 160 ± 42 | 0.21 | 0.02 |
| Right ventricular diastolic volume index (ml/m 2 ) | 68 ± 11 | 73 ± 17 | 69 ± 15 | 80 ± 17 | 0.35 | 0.03 |
| Right ventricular systolic volume (ml) | 59 ± 12 | 70 ± 26 | 61 ± 14 | 89 ± 36 | 0.11 | <0.001 |
| Right ventricular systolic volume index (ml/m 2 ) | 32 ± 7 | 36 ± 12 | 31 ± 7 | 42 ± 8 | 0.20 | <0.001 |
| Right ventricular ejection fraction (%) | 54 ± 5 | 51 ± 11 | 52 ± 6 | 39 ± 15 | 0.18 | 0.04 |
| Late gadolinium enhancement (n) | 0 (0%) | 3 (7%) | 1 (3.5%) | 2 (14%) | 0.55 | 0.25 |
| Extracellular volume fraction | 0.28 ± 0.02 | 0.36 ± 0.03 | 0.36 ± 0.02 | 0.38 ± 0.03 | <0.001 | 0.03 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


