Improvements in fetal echocardiography have increased recognition of fetuses with congenital heart disease (CHD) that require specialized delivery room (DR) care. In this study, care protocols for these low-volume and high-risk deliveries were created. Elements included (1) diagnosis-specific DR care plans and algorithms, (2) a multidisciplinary team with expertise, (3) simulation, (4) checklists, and (5) debriefing. The purpose of this study was to assess the accuracy of fetal echocardiography to predict the need for specialized DR care and determine the effectiveness of the care protocols for the treatment of patients with critical CHD. Fetal and postnatal medical records and echocardiograms of fetuses with CHD assigned to an advanced level of care were reviewed. Safety and outcome variables were analyzed to determine care plan and algorithm efficacy. Thirty-four fetuses were identified: 12 delivered at Children’s National Medical Center and 22 at the adult hospital. Diagnoses included hypoplastic left heart syndrome, aortic stenosis, d-transposition of the great arteries, tetralogy of Fallot with absent pulmonary valve, complex pulmonary atresia, arrhythmias, ectopia cordis, and conjoined twins. Delivery at Children’s National Medical Center was associated with a shorter time to specialty care or intervention. Measures of physiologic stability and survival were similar. Need for specialized care was predicted in 84% of deliveries. For hypoplastic left heart syndrome, intervention was predicted in 10 of 11 deliveries and for d-transposition of the great arteries in 10 of 12 deliveries. Care algorithms addressed most DR events. Of the unanticipated events, none were unrecoverable. DR survival was 100%, and survival to discharge was 83%. In conclusion, fetal echocardiography predicted the need for specialized DR care in fetuses with critical CHD. Algorithm-driven protocols enable planning such that maternal and infant risk is minimized and outcomes are good.
Advances in fetal echocardiography using high-resolution ultrasound and serial imaging have led to an increased number of fetuses diagnosed with congenital heart disease. Clinical course in utero and at delivery can now be predicted, and as a consequence, fetal medicine specialists are being asked to consider the fetus as a patient and the transition to postnatal life an important part of care. Although several studies suggest that fetal diagnosis improves outcomes for patients with severe congenital heart disease by preventing postnatal instability, certain populations of patients with congenital heart disease continue to have high mortality rates due to compromise that begins in the delivery room (DR). Newborns with hypoplastic left heart syndrome (HLHS) or d-transposition of the great arteries (d-TGA) with restrictive foramen ovale or intact atrial septum, tetralogy of Fallot with absent pulmonary valve, Ebstein’s anomaly, or arrhythmias with hydrops have all been reported to have poor outcomes if diagnosed prenatally. For these fetuses, subspecialty care must begin in the DR to affect morbidity and improve survival. Some free-standing children’s hospitals have incorporated delivery suites to accommodate high-risk deliveries, but expectant women may have increased risk if deliveries are performed at facilities that rarely provide this service. Using published data and clinical experience, we developed a risk stratification system for delivery of fetuses with congenital heart disease. For fetuses with little or no risk for requiring specialized DR care, delivery at the local hospital is recommended. If it is anticipated that specialized care or intervention will be required, delivery at Children’s National Medical Center (CNMC) or the adjacent adult hospital with CNMC specialists in the DR is planned. For these complex deliveries, a “complex care for in utero to birth” (CCUB) team was created to oversee these low-volume and high-risk deliveries. The purpose of this study was to assess the accuracy of fetal echocardiography to predict need for specialized DR care and determine the effectiveness of CCUB protocols for the treatment of newborns with critical congenital heart disease. We hypothesized that the identification of fetuses with critical congenital heart disease and the institution of disease-specific care protocols would improve outcomes in this high-risk group of patients.
Methods
We retrospectively reviewed the records of all fetuses with critical congenital heart disease evaluated from November 2004 to March 2012. Fetuses were identified by searching the Fetal Heart Program database. Fetal and postnatal echocardiograms and maternal and infant medical records were reviewed, and data were collected after all patient identifiers were removed. All decisions regarding DR care plans were made according to clinical practice. This study was approved with a waiver of the requirement for informed consent by the hospital institutional review board.
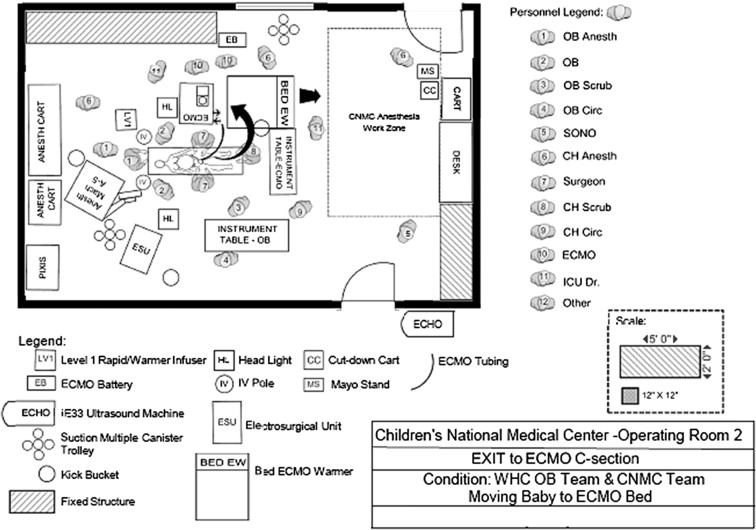
A risk-stratified care system using fetal echocardiographic data was created to predict the occurrence of hemodynamic instability at delivery for fetuses with congenital heart disease. For each anticipated level of care (LOC), recommendations are prepared for DR and postnatal management. Key components include DR staffing, delivery location, and cardiac-specific DR care ( Table 1 ). Low-risk fetuses are delivered at the local hospital with outpatient subspecialty follow-up (LOC 1) or are stabilized using standard neonatology DR practices with subsequent transport to CNMC for subspecialty care (LOC 2). For high-risk fetuses, a coordinated delivery with a multidisciplinary, specialized CNMC care team present in the DR is planned. In instances in which specialized medical care is required and intervention is not anticipated (LOC 3), delivery at the adjacent adult hospital, the Washington Hospital Center, is planned. If it is anticipated that an immediate surgical or catheter intervention is required (LOC 4), every effort is made to deliver in the CNMC operating room. For LOC 4 deliveries, DR care planning includes the use of an improvement science approach for the creation of disease-specific care protocols, including (1) multidisciplinary team assignment with subspecialty-driven expertise, (2) DR simulation, (3) the creation of DR checklists, and (4) debriefing. Individualized care algorithms are created for LOC 4 patients delivered at CNMC ( Figure 1 ). In addition, operating room maps are made to document staff and equipment position during all phases of care ( Figure 2 ). The multidisciplinary team includes experts in obstetrics, anesthesia, cardiology, neonatology, surgery, and nursing. Simulation involves participation from all DR personnel. Two simulations are planned: the first at completion of the plan of care and the second the evening before delivery, with all equipment in place. The care algorithm is enacted, including all potential branch points from what is expected. Specialty-specific checklists for each phase of the delivery, including (1) planning, (2) prelaunch (before the woman is brought into the DR), (3) launch (initiation of procedure), (4) delivery, and (5) recovery, are reviewed to ensure that all personnel are ready. Postdelivery debriefing occurs with team members within 1 week after the delivery to discuss events. A summary document with recommendations is generated.
| LOC | Expected Physiology | Example CHD | Delivery Recommendations | Cardiac DR Recommendations |
|---|---|---|---|---|
| 1 | No instability expected in first weeks of life | ASD, VSD, AVSD, mild valve disease | Delivery at local hospital | Arrange outpatient follow-up |
| 2 | Stability in DR expected but requiring postnatal catheterization or surgery | Ductal-dependent lesions including HLHS, PA/IVS, severe TOF | Planned vaginal induction at about 39 weeks at local hospital; transport to CNMC | Neonatologist in DR; PGE if indicated |
| 3 | Instability requiring immediate specialty care in DR before catheterization or surgery | HLHS or TGA with RFO, CHD or arrhythmia with decreased heart function | Planned vaginal induction at WHC at about 38–39 weeks with “bailout” to C/S if necessary | CNMC specialists in DR; medications predetermined by care plan |
| 4 | Instability requiring immediate catheterization or surgery in DR | HLHS or TGA with severe RFO or IAS, CHD or arrhythmia with hydrops | Planned C/S at CNMC usually at 38 weeks | Multidisciplinary specialized care team in DR; medications and equipment predetermined by care plan, including ECMO if indicated |

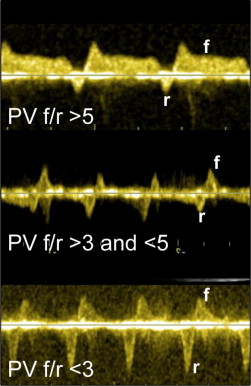
Determination of disease severity requiring LOC 3 or 4 was established using published fetal echocardiographic criteria when available ( Table 2 ). In cases for which criteria were lacking, multidisciplinary conferencing with consensus determined the need for advanced LOC. The final fetal echocardiogram before delivery was used to refine DR planning. Fetal data collection included initial cardiac diagnosis and diagnosis at final evaluation. Fetuses with HLHS and d-TGA were analyzed separately. For HLHS, atrial septal anatomy was noted and the pulmonary vein forward/reversed velocity-time integral ratio (PV f/r) documented. The lower the ratio, the greater the reversed flow in the pulmonary veins from presumed left atrial hypertension ( Figure 3 ). For d-TGA, the atrial septal anatomy was described as hypermobile, tethered with abnormal angle, bowing, or intact ( Figure 4 ). The ductus arteriosus was measured, and abnormal flow patterns (reversed, bidirectional, or accelerated) were documented. For fetuses with severe arrhythmias, arrhythmia progression was noted. For all, changes in qualitative cardiac function or the onset of hydrops were noted. Anticipated delivery plan details, including place and mode of delivery, intervention expected, and LOC 4 care algorithms, were collected. Postnatal data, including gestational age, place and mode of delivery, cardiac diagnosis, time to initial care and intervention, and intervention performed, were collected. Arterial blood gas and lactate measurements in the first 24 hours were collected as markers of physiologic disturbance. Outcome measures included DR survival and survival to discharge. Maternal outcome measures included delivery complications, survival, and time to discharge.
| Diagnosis | LOC 3 | LOC 4 | Care Plan |
|---|---|---|---|
| HLHS with RFO or IAS | PV f/r <5 and >3 (5) | PV f/r <3 (6) | LOC 3: PGE, catheterization pending confirmation of RFO in DR LOC 4: PGE, immediate catheterization |
| TAPVR (obstructed) | N/A | Accelerated flow in connecting vein | Consider ECMO |
| TGA with RFO or IAS | Hypermobile (7) or tethered atrial septum or angle of septum primum <30° (8,9) ∗ | Atrial septal bowing >50% into LA, IAS, or RFO with abnormal ductus (z score <2 and/or abnormal flow) (8,9) | LOC 3: PGE, plan for BAS in cardiac ICU LOC 4: PGE, plan for BAS in DR PHTN therapy if abnormal ductus |
| Severe TOF/APV | Dilated heart with decreased function, significant arrhythmias | Hydrops, CLE on fetal MRI (24) | Specialized ventilation, consider ECMO |
| Severe Ebstein’s anomaly | Dilated heart with decreased function, significant arrhythmias | Hydrops | Therapy to decrease PVR and improve function With hydrops, consider early delivery |
| Tachyarrhythmias | Uncontrolled arrhythmia with decreased heart function | Hydrops | Cardioversion or medical therapy in DR If hydrops, consider early delivery |
| Complete heart block | Decreased CVP score to <7 (20), ventricular rate <55 with decreased function | Hydrops | Pacing in DR vs medical chronotrope If hydrops, consider early delivery |
∗ Criteria for LOC 4 planning updated to include these given experience with this group.

Prenatal LOC assignment was compared with postnatal diagnosis and outcome to determine the accuracy of fetal diagnoses to predict postnatal events. Sensitivity and specificity were calculated. Congruence between anticipated and actual events was determined for LOC 4 patients by comparing delivery algorithms to actual events. Determination was made as to whether unplanned events were recoverable or resulted in significant morbidity by review of debriefing notes. Statistical analyses were developed to evaluate outcomes on the basis of LOC, which was closely related to the decision regarding site of delivery. Three types of outcomes were assessed: (1) time events, including initiation of care, intervention, and discharge; (2) blood gas results; and (3) frequency of complications or unplanned events. For time-event outcomes, Kaplan-Meier analyses were performed. Differences in the frequency of events were assessed using contingency table analyses. Differences in mean blood gas parameters were evaluated using analysis-of-variance models. Analyses were treated as hypothesis generating rather than hypothesis testing, and therefore more attention was paid to the magnitude and range of differences rather than to the level of statistical significance.
Results
Thirty-four fetuses in 33 pregnancies were identified to have congenital heart disease requiring specialized DR care. Diagnoses included HLHS and variants with restrictive foramen ovale or intact atrial septum (n = 11), critical aortic stenosis with left ventricular dysfunction (n = 2), d-TGA with restrictive foramen ovale or intact atrial septum and/or abnormal ductus arteriosus (n = 12), tetralogy of Fallot with absent pulmonary valve with lobar emphysema of the right lung (n = 1), complex pulmonary atresia with sinusoids and restrictive foramen ovale (n = 1), complete heart block with low ventricular rate and ventricular dysfunction (n = 2), complex arrhythmia with long pauses and periods of torsades de pointes (n = 1), ectopia cordis (n = 2; 1 with tricuspid atresia/pulmonary stenosis and 1 with the lower sternum compressing the ventricles), and conjoined twins with thoracopagus (n = 1; livers joined, hearts separate; 1 twin with severe tetralogy of Fallot, the other with atrioventricular canal defect). Nineteen fetuses were assigned LOC 4, and 15 (2 conjoined twins) were assigned LOC 3. Twelve of 19 LOC 4 fetuses (63%) were delivered at CNMC. All 15 LOC 3 fetuses and 7 of 19 LOC 4 fetuses (37%) were delivered at Washington Hospital Center. Diagnoses, interventions, and outcomes for LOC 3 and 4 patients are summarized in Figure 5 . Fetal echocardiographic features as they relate to intervention and outcomes for fetuses with HLHS and d-TGA are summarized in Figure 6 .

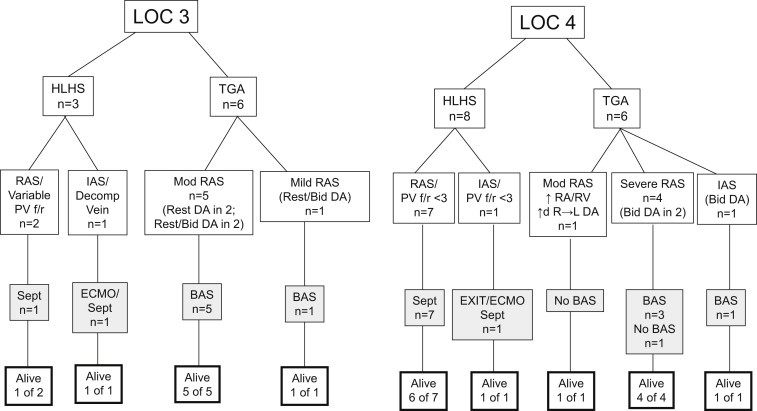
All fetuses assigned LOC 3 were delivered at Washington Hospital Center with a CNMC specialist in the DR (cardiologists (n = 10), cardiac intensivists (n = 2), electrophysiologist (n = 3), and transport team members (n = 15)). Eight (53%) were determined at delivery to require emergent intervention: 2 with HLHS and 6 with d-TGA. In 1 fetus with HLHS, PV f/r was 1.5; however, a decompressing vein supplied adequate egress of blood from the left atrium in utero. In another with HLHS, a restrictive foramen ovale was appreciated, but PV f/r was difficult to interpret given that 1 vein had PV f/r >5 and another suggested need for intervention with PV f/r of 1.7. Immediate postnatal evaluation led to determination that catheter intervention was warranted, and the 2 patients were transferred from the DR to the catheterization laboratory. All patients with HLHS underwent Norwood palliation with Sano modification; 2 of 3 survived. In the 6 fetuses with d-TGA, in utero findings included a hypermobile septum in 1 and tethered septum in 5. The ductus arteriosus was small in 5 fetuses, and bidirectional flow was noted in 3. For all, hypoxia was noted in the DR, echocardiography confirmed severe atrial septal restriction, and immediate transport from the DR to cardiac intensive care unit for balloon atrial septostomy occurred. All underwent arterial switch and survived. Seven fetuses did not require immediate DR intervention but did undergo catheterization and/or surgery. The patients with complete heart block underwent pacemaker implantation. The patients with pulmonary atresia and HLHS underwent surgical palliation. The patient with aortic stenosis underwent aortic valvuloplasty. All survived. The conjoined twins were separated but did not survive.
Twelve fetuses assigned LOC 4 were delivered at CNMC, and 7 were delivered at Washington Hospital Center with CNMC specialists and transport team members present in the DR. The reasons for delivery at Washington Hospital Center included early onset of labor resulting in emergent delivery in 4 and maternal refusal of cesarean section in 3. Sixteen of 19 fetuses (84%) assigned LOC 4 required immediate intervention (9 born at CNMC, 7 at Washington Hospital Center). Seven of 8 with HLHS were taken from the DR to the catheterization laboratory and underwent atrial septostomy. Of these, 1 was placed on extracorporeal membrane oxygenation (ECMO) before septostomy because of hemodynamic instability and an interrupted inferior vena cava that complicated access for the septostomy. One fetus with HLHS and intact atrial septum underwent an ex utero intrapartum therapy (EXIT) to ECMO procedure in the DR. Atrial septostomy was performed in the catheterization laboratory. All 8 fetuses underwent Norwood/Sano operations; 7 survived. The patient with aortic stenosis and restrictive foramen ovale underwent atrial septostomy. Aortic valvuloplasty was not performed given significant aortic insufficiency. The infant died after the procedure. Of the 6 patients with d-TGA, 5 had either a severely restrictive foramen ovale with septal bowing or intact atrial septum. Of these, 3 also had abnormal ductal flow. One had a tethered septum with dilated right atrium and ventricle and restrictive ductus arteriosus with accelerated right-to-left flow (after initial study with bidirectional flow). All were successfully treated in the DR with prostaglandin infusion and ventilatory measures to decrease pulmonary vascular resistance. Four of the 6 required balloon septostomy. All underwent arterial switch and survived. The infant with ectopia cordis and congenital heart disease was born severely cyanotic and required significant resuscitation. Stabilization was achieved with paralytics, sedation, and aggressive ventilation in addition to prostaglandin. The infant subsequently underwent a complicated surgical reconstruction. The second with ectopia cordis developed frequent premature ventricular contractions that did not require therapy. The chest was closed during omphalocele repair. The fetus with tetralogy of Fallot with absent pulmonary valve was hypoxic in the DR. Upon arrival in the cardiac unit, worsening hypoxemia and poor cardiac output from air trapping led to urgent cannulation onto ECMO, which was anticipated as part of the care plan. Surgery was performed on day 2 of life and the ECMO circuit removed. The patient with torsades de pointes required aggressive medical therapy in the DR, including magnesium, lidocaine, and esmolol. A pacer/defibrillator was ultimately placed.
Need for emergent care and intervention was predicted in 84% of fetuses assigned LOC 4. In 53% of LOC 3 fetuses, emergent intervention occurred but was not predicted. Overall, assignment of LOC 4 predicted need for emergent care/intervention with sensitivity of 67% and specificity of 70%. Subgroup analysis of patients with HLHS revealed that LOC 4 assignment using PV f/r <3 predicted the need for intervention with sensitivity of 80% and specificity of 100%. For 3 fetuses with HLHS, although PV f/r was <3, additional findings led to LOC 3 assignment. In 1 with HLHS and intact atrial septum, a decompressing vein, noted to be patent in utero, became obstructed with ventilation. This patient required intervention. In 2 fetuses, differing PV f/r flow in the right and left veins suggested partial obstructed pulmonary venous flow. Postnatally, in both, a single right pulmonary vein drained into the low-pressure right atrium because of posterior deviation of septum primum. One of 2 required intervention. For d-TGA fetuses, LOC 4 assignment predicted need for septostomy with a sensitivity of only 40% and specificity of 0%. In our initial experience, given the need for cesarean section with a CNMC delivery, only fetuses with severely restrictive foramen ovale or intact atrial septum were assigned LOC 4. In 1 fetus, an exception was made given significant right atrial and ventricular dilation and accelerated ductal flow suggesting abnormal pulmonary vascular resistance and ventricular dysfunction in the presence of a moderately restrictive foramen ovale. Two of 4 required intervention. The 6 fetuses with d-TGA assigned LOC 3 had only mild or moderate foramen ovale restriction. All required septostomy. Of note, 10 of 11 fetuses (91%) with HLHS assigned to LOC 3 or 4 had an intact atrial septum or restrictive foramen ovale critical enough to undergo catheter intervention. Similarly, 10 of 12 (83%) with d-TGA assigned LOC 3 or 4 due to the presence of any foramen ovale findings required septostomy. Given that LOC 3 or 4 assignment prompted delivery planning that included a specialist in the DR and immediate transport with the intervention team ready, in no case was the team unprepared. Furthermore, review of fetuses assigned LOC 2 during the same time period found 39 with HLHS and 7 with d-TGA. None required emergent intervention. Using the distinction of LOC 2 for fetuses anticipated to require no specialized care and delivery at the local hospital, and LOC 3 or 4 for those requiring specialty care including being prepared for intervention, fetal echocardiographic risk stratification predicted the need for intervention in fetuses with HLHS with sensitivity of 100% and specificity of 98% and in those with d-TGA with sensitivity of 100% and specificity of 78%.
Thirty-three fetuses underwent multiple studies. In the 11 with HLHS, the initial study was at a median of 24 weeks (range 17 to 31) and the final study at 37 weeks (range 32 to 39). PV f/r flow worsened in all; mean PV f/r was 3.8 on the initial study and 1.8 on the final study (p = 0.005). On the initial study, PV f/r was >5 in 3 fetuses, <5 and >3 in 4, and <3 in 4. Final PV f/r was <3 in all. In 7 fetuses, PV f/r change from the first to the final study resulted in an LOC increase from 3 to 4. For the 12 fetuses with d-TGA, the initial study was at a median of 24.6 weeks (range 19 to 34) and the final study at 36.5 weeks (range 32 to 39). Atrial septal restriction increased in 10 fetuses; in 1, it remained the same, and in 1, there was a single study. On the final study, 1 fetus had a hypermobile septum, 6 had a tethered septum, 4 had a bowing septum, and 1 had an intact atrial septum. Ductus arteriosus flow changes occurred in 6 fetuses. In 9, the ductus arteriosus was abnormal at the final study. The ductus was small in 6 fetuses; flow was bidirectional in 6, and accelerated in 1. In 5 fetuses, the foramen ovale became severely restrictive or intact by final study. In 6, foramen ovale and/or ductus arteriosus characteristics worsened such that LOC increased from 3 to 4. In the fetus with tetralogy of Fallot with absent pulmonary valve, there was progressive right ventricular dilation, and in 1 fetus with aortic stenosis, there was progressive left ventricular dilation and onset of hydrops. For the 2 fetuses with complete heart block, there was a decrease in heart rate and development of pericardial effusion, and in the 1 with complex arrhythmia, on final evaluation, heart function was decreased, and the rhythm showed periods with heart rates >300 beats/min and pauses of 1.5 seconds. Given the timing of the findings, no in utero therapy was initiated. In all others, there was no change from the first to the final study. In no fetus did any characteristics improve.
Comparison data of deliveries that occurred at CNMC versus Washington Hospital Center are listed in Table 3 . CNMC delivery was associated with a shorter median time to specialty care and intervention. In addition, there was a narrower range in time to intervention or catheterization, suggesting less variability in care. DR survival was 100%, and length of stay and survival were similar. Analysis of blood gas data revealed that mean oxygen level was higher in the group delivered at CNMC; however, there were no differences in pH or lactate. For those requiring intervention, delivery at CNMC versus Washington Hospital Center was associated with a shorter time to care (1 vs 9 minutes, p = 0.0001). Median time to intervention was similar (p = 0.19), although ranges were again narrower in the CNMC group. There was no difference in DR survival. Length of stay trended toward being longer (39 vs 26 days, p = 0.09), and survival trended toward being lower (88% vs 100%, p = 0.06) for those delivered at CNMC (these data may be biased in that fetuses delivered at CNMC in general had more severe disease). Analysis of blood gas data revealed that mean oxygen level was again higher in the group delivered at CNMC (30.9 vs 22.6 mm Hg, p <0.05). Mean pH and lactate were not different. For fetuses that required no intervention, there were no differences in any parameters. In subgroup analyses of neonates with HLHS and d-TGA, there were no differences in blood gas data between CNMC and Washington Hospital Center delivery.



