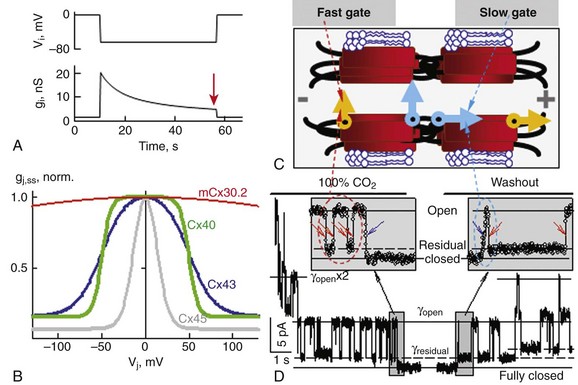
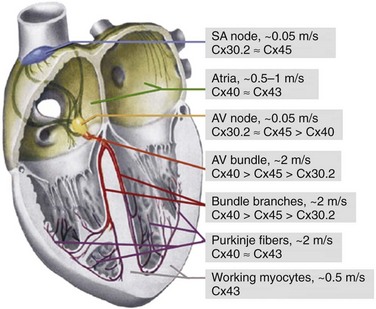
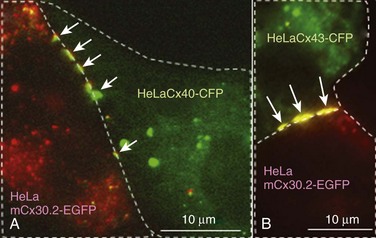
8 Connexins Expression and Oligomerization De novo Formation of Gap Junction Channels Voltage and Chemical Gating of Connexin-Based Channels Hemichannels: Function and Physiologic Relevance Connexins Expression Pattern and Propagation of Excitation in the Heart Cell-Cell Coupling and Electrical Anisotropy in the Heart. Alterations of Cell-Cell Coupling in Cx-Deficient Animals and Disease-Related Mutations Connexins (Cxs), a large family of homologous membrane proteins, form gap junction (GJ) channels that provide a direct pathway for electrical and metabolic signaling between cells. Gap junctional communication is critically important in the spread of excitation in the heart, communication between neurons and glia, metabolic exchange between cells in the lens and other tissues lacking blood circulation, organ formation during development, and regulation of cell proliferation.1 Each GJ channel is composed of two hemichannels (connexins). Oligomerization of six Cxs into hemichannels starts in the endoplasmic reticulum and is completed in the Golgi, from where vesicles approximately 100 to 150 nm in diameter containing hemichannels travel to fuse with the plasma membrane. Cxs are predicted to have four alpha-helical transmembrane domains (M1 to M4), intracellular N- and C-termini (NT and CT), two extracellular loops (E1 and E2), and a cytoplasmic loop (CL).2 The density map of the crystal structure of human Cx26 at 3.5 Å resolution revealed that M1 and EI line the channel pore, which is narrowed by six NT domains residing in the channel vestibule. These structural data were supported by functional studies using a cysteine scanning approach3 demonstrating that NT, M1, and E1 domains are directly involved in defining GJ channel unitary conductance, gating properties and permselectivity.4 Thus far, at least 21 Cx genes have been identified in humans. GJ channel de novo formation was examined by manipulating two separate cells into contact while monitoring electrical cell-cell coupling. This approach has been used to study formation of GJs between cells of Xenopus blastulae and myoballs, rat cardiomyocytes, insect cells, and HeLa cells transfected with Cx40.5 Most likely, the initial step in this process is the formation of hemichannel plaques (hemi-plaques) in the plasma membrane,6,7 which should stabilize the hemichannels’ stochastic lateral diffusion in the plasma membrane and create an outward-directed curvature that should reduce the distance between hemichannels in apposing cells.8 Theoretical studies show that when five or more proteins aggregate, the cluster becomes stable despite their pairwise repulsions.8 Subsequent steps likely include a close apposition between cells, possibly mediated by adhesion molecules and overlapping of hemi-plaques that could lead to formation of precursors of GJ channels termed formation plaques (FPs), which gradually transform into junctional plaques (JPs).9 Alignment and docking of apposed hemichannels comes thereafter, followed by the formation of a high-resistance seal to isolate the nascent channel pore from the extracellular space, ending with the channel pore opening. Docking of individual hemichannels furthermore stabilizes FPs and reduces the distance between still undocked hemichannels, which may explain an acceleration of de novo formation kinetics after functional appearance of the first channels.10 De novo formation of GJ channels, their clustering into JPs, as well as GJ channels’ turnover and their gating was shown to be regulated by different intracellular pathways that involve cAMP, G proteins, and phosphorylation.11 Significant progress has been made in understanding Cx trafficking, JP formation, and internalization using cells expressing Cxs fused with color variants of green fluorescent protein (GFP) or containing a tetracysteine tag.12,13 JPs serve as “crystallization centers,” attracting laterally moving hemichannels and catalyzing their docking due to close apposition of hemichannels in the area of JPs. By combining fluorescence imaging of Cx-GFPs with dual whole-cell voltage clamp recording, it was shown that only cell pairs that exhibit a JP are electrically coupled.14 Furthermore, it was shown that only a small fraction of channels assembled in a JP are functional at a given time and this ratio varies from approximately 0.01 for Cx45 and Cx57 to 0.1 for Cx43.15,16 This fraction can be modulated in the time scale of a few minutes by pH, long-chain alkanols, [Mg2+]i, and many other chemical factors.15,17 This indicates that this type of modulation does not involve an assembly of new GJs or their turnover. Cell-cell communication can be organized through homotypic (same Cx isotype in both hemichannels), heterotypic (2 Cx isotypes form GJ channels, but each hemichannel is assembled from 1 isotype) and heteromeric (different Cx isotypes at least in one of the hemichannels) channels that vary in conductance, permselectivity, and gating properties. Formation of heterotypic and heteromeric GJ channels can occur in the heart, particularly in the conduction system, where cardiomyocytes co-express several Cx isoforms. Most heterotypic junctions that can be formed in the heart exhibit asymmetric voltage gating and rectification of the current voltage (I-V) relation.18,19 Asymmetric junctional conductance to transjunctional voltage (gj-Vj) dependence can result in impeded signal transfer in one direction and facilitated transfer in the opposite direction, as it was demonstrated for Cx43/Cx45, Cx31/Cx45, and Cx40/Cx45.5,20 This may facilitate an initiation of one-directional block, which is critical in reentry formation. The existence of multiple Cxs raises the questions of how they differ and how they interact. Unitary conductances of GJs formed of Cx isoforms range from approximately 10 pS (e.g., mCx30.2) to 300 pS (e.g., Cx37) and channels vary in permselectivity from being nonselective to preferentially selective for cations or anions.1,21 A property that appears to be common to GJs formed by any Cx isoform is sensitivity of gj-Vj. Figure 8-1, A shows a decay of gj until a steady-state level (gj,ss) reached (as indicated by the arrow) in response to Vj step voltage. The symmetric reduction in gj,ss with positive or negative Vj has been explained by having identical Vj-sensitive gate(s) in each apposed/junctional hemichannel (aHC) of the GJ channel.23 Normalized gj,ss-Vj dependencies show big differences in sensitivity to Vj among four principal Cxs of cardiomyocytes (see Figure 8-1, B). A common feature of Vj-gating is that gj,ss does not decline to zero with increasing Vj, but instead reaches a residual conductance termed gmin. Single-channel studies have shown that gmin is due, at least in part, to incomplete closure of the GJ channel by Vj, which causes channels to close to a subconductance (residual) state with fast gating transitions (~1 ms or less).24 It was shown that Vj as well as chemical uncouplers can also induce gating transitions to the fully closed state and that these transitions are slow, approximately 10 ms. Gating to different levels via fast and slow gating transitions led to the suggestion that there are two distinct Vj-sensitive gates, termed fast and slow or loop.5 The fast gate closes channels to the residual state, and the slow gate closes them fully (see Figure 8-1, C). Figure 8-1, D, shows an example of fast and slow gate operation during acidification with carbon dioxide (CO2), which gradually reduced the number of operating channels. When only one channel was left to operate, the fast gate was governing fast transitions between open and residual states, finally being closed by the slow gate with transitions lasting approximately 10 ms. The opposite sequence of events was observed during washout from CO2. Two distinct gating mechanisms were also demonstrated in Cx-based unapposed/nonjunctional hemichannels (uHC).25 Figure 8-1 Gating of GJ channels. A, Typically, junctional conductance (gj) measured between cell pairs decays in response to transjunctional voltage (Vj), reaching some steady-state level (gj,ss) (arrow). B, Normalized gj,ss dependencies on Vj show big differences in sensitivity to Vj among four principal Cxs of cardiomyocytes. C, A schematic of the GJ channel containing fast and slow gating mechanisms. The fast gate (orange) closes the channel partially, whereas the slow gate (blue) closes the channel fully. D, The effect of CO2 on voltage gating at the single-channel level in a cell pair of fibroblasts expressing Cx43. Exposure to CO2 caused full uncoupling. Ij was monitored at Vj = 55 mV just before full uncoupling and at the beginning of CO2 washout. Channels exhibited two types of Ij transition: (1) between open and residual state (~90 pS), with a transition time of approximately 1 ms (red arrows); and (2) between open and fully-closed states (~120 pS), with a transition time of approximately 10 ms or more (blue arrows). The signals in the insets (sampled at 1-ms interval) illustrate that the last channel closes with a transition time of approximately 10 ms and the first channel opens with a transition time of approximately 19 ms. The slow opening of the first channel during washout was followed by fast flickering between the open and residual states. When two operating channels were in the residual state, gj equals the sum of two γres (dashed lines). (Modified from Bukauskas FF, Peracchia C: Two distinct gating mechanisms in gap junction channels: Co2-sensitive and voltage-sensitive. Biophys J 72:2137–2142, 1997.) Mutational studies revealed that the gating polarity of the fast gating mechanism is governed by charged residues in the N-terminal domain and that this polarity could be reversed independently from the slow gating mechanism. Modifications of Cx43, including deletion of the CT domain26 or attachment of aequorin or enhanced green fluorescent protein (EGFP) to CT, selectively abolish fast gating to the residual state.5 These data demonstrate that in each hemichannel there are two molecularly distinct gating mechanisms that require further study in determining Cx domains functioning as gating and sensorial elements for each gate. This hypothesis has received support from studies performed in channels formed of Cx30, Cx31, Cx32, Cx37, Cx40, Cx43, Cx45, and Cx57,1,5 suggesting that fast and slow gates are likely to be common to all Cx isoforms. Gating properties of GJ channels can be described using the Boltzmann function,23 assuming that GJ channels have two states, open and fully closed, like most ionic channels, and that each hemichannel gates independently. The accumulation of data demonstrating gating to the substates and two distinct gating mechanisms stimulated the development of gating models that more intimately describe GJ channel gating.27,28 Recently, a stochastic 16-state model (S16SM) of Vj-gating was developed, in which each aHC contained both fast and slow gating mechanisms.24 The model can be used to simulate Vj-gating in homotypic and heterotypic junctions. A S16SM allows for simulation of Ij dynamics as well as the gj,ss-Vj plot of GJs depending on individual gating parameters of four gates. The model includes more than 12 parameters characterizing certain gating properties of GJs, and to evaluate them manually from experimental gj,ss-Vj dependencies would resemble the search for “the needle in the haystack.” To automate this fitting process, we have adopted the Global Coordinate Optimization (GCO) algorithms,24 which are based on the Bayesian approach to filter the stochastic component and smooth small local minima while searching for the global minimum. GCO of experimental data can be performed using the online version of the algorithm at http://connexons.aecom.yu.edu. All GJ channels also display chemical gating, which shares several features with the slow voltage sensitive gate, (i.e., it reduces gj to zero and closes channels fully.)1,5 We assume that asynchrony of conformational changes in each of six Cx “subgates” of the slow gate leads to multistep gating transitions lasting approximately 10 ms or more. This may be caused by a low level of cooperativity between these “slow subgates.” Similar slow transitions were observed at the initial stages of channel pore opening during de novo channel formation and during gating induced by transmembrane voltage (Vm) or chemical uncouplers, as illustrated in Figure 8-1, D. Although fast gating to the residual state is induced exclusively by Vj, the slow gating mechanism can be triggered by Vj, Vm, and chemical factors. Interestingly, uncoupling induced by alkanols, Ca2+, and H+ was shown to be partially reversible by changes in Vm or Vj.5 These data suggest that chemical- and voltage-sensitive gating mechanisms interact and may share the common gating element of the slow gate, which is triggered by sensorial elements specific for voltage and different chemical agents. Surface expression of uHCs has been demonstrated in several cell lines and primary cultures by electron microscopic, biochemical, and electrophysiologic methods.29,30 It has long been thought that hemichannels remain closed until docking with hemichannels from an apposed cell because ions and metabolites can leak the cell through open hemichannels. Furthermore, Cx43 uHCs have been implicated in diverse roles in cell physiology and pathophysiology, including volume regulation; efflux of glutamate, NAD+, cAMP, IP3, and ATP acceleration of astrocytes and cardiomyocyte death during metabolic inhibition, and transduction of extracellular signals regulating apoptosis development.31,32 The opening of hemichannels can be enhanced by cell polarization to positive potentials and reduction of Mg2+ and Ca2+ in the extracellular milieu.33 Conversely, closure of hemichannels can be induced by low intracellular pH, 18-α-glycyrrhetinic acid, trivalent cations (La3+ or Gd3+), and some chloride channel blockers.31,34 Typically, at the single-channel level, the I-V relation of uHCs rectifies and their unitary conductances are approximately twice the conductances of the GJ channel formed of the same Cxs.35 Lately, Cx43 hexamers/hemichannels were shown in purified mitochondrial preparations of the mouse myocardium, which may contribute to mitochondrial K+ uptake.36 More detailed studies revealed that Cx43 is expressed in subsarcolemmal, but not interfibrillar, mitochondria, and it was proposed that Cx43 plays a critical role in mediating the cardioprotective function of ischemic preconditioning.37,38 It is hypothesized that preconditioning leads to opening of Cx43 hemichannels in the inner mitochondrial membrane, with subsequent loss of ATP from the mitochondrial matrix. This activates ATP-dependent K+ channels that appear to be central to the protective effect.39 It has been shown that among cardiac Cxs, mCx30.2,29 Cx43,35 and Cx4540 form functional uHCs, whereas Cx40 does not.41 Typically, uHCs tend to open at higher positivity on the cytoplasmic side. Presumably, open channel probability of uHCs increases during action potentials (APs) and to a greater extent during tachycardia when cells are depolarized for a longer time. Opening of hemichannels in nodal cells would depolarize them and locally increase the extracellular concentration of K+ ions. Depolarization could inactivate inward currents driving excitability and contribute to a reduction in conduction velocity. This hypothesis raises a new mechanism explaining a long atrioventricular (AV) delay as a result of mCx30.2 hemichannels function.42 This corroborates with a reduction in PQ-interval in mCx30.2 knockout mice.43 Another class of membrane proteins, pannexins (Panxs), which also form uHCs but not GJ channels, are involved in paracrine signaling and mechanosensitivity.44 It was shown recently that Panx1 constitutes 300 pS uHCs in cardiomyocytes, suggesting that their opening can depolarize the plasma membrane and trigger premature APs that may lead to arrhythmogenic activities.45 To date, four connexins—mCx30.2, Cx40, Cx43, and Cx45—are known to be expressed by cardiomyocytes and form GJ channels, mediating the spread of excitation in the heart, as well as direct intercellular metabolic communication42; human ortholog of mCx30.2 is Cx31.9.19 Expression patterns of all four cardiac Cxs are shown in Figure 8-2. In the mammalian heart, the cardiac impulse is initiated in the spontaneously active pacemaker cells of the sinoatrial (SA) node, in which mCx30.2 and Cx45 GJs integrate thousands of pacemaker cells with various intrinsic frequencies of excitation into one functional unit. These Cxs form homotypic GJ channels with conductances of 9 pS and 32 pS, respectively, as well as mCx30.2/Cx45 heterotypic GJs with the single-channel conductance of approximately 17 pS,5,19 or even heteromeric GJs, which can exhibit a variety of single-channel conductances in a range of about 9 pS to 32 pS.46 Electrical coupling in the transitional region between the SA node and the atrium should not be too high, thus preserving functional identity of pacemaker cells, but at the same time allowing for signal transfer. Figure 8-3 shows images of cell pairs formed of HeLaCx30.2-EGFP (red) with HeLa cells expressing Cx40 tagged with cyan florescent protein (CFP) (green, part A) and Cx43-CFP (part B). Both heterotypic junctions exhibited extensive formation of JPs (arrows) and Vj-gating asymmetry typical for most of heterotypic junctions.19 Figure 8-2 Schematic of the heart with indications showing connexin expression patterns and conduction velocities in different regions of the heart. (Modified from Netter: Atlas der Anatomie des Menschen, ed 4, New York, 1993, Ciba Geigy Corp.) Figure 8-3 Formation of heterotypic GJs visible as multiple junctional plaques (yellow) indicated by arrows and located in between cells expressing mCx30.2-EGFP (red) and those expressing Cx40-CFP (A) or Cx43-CFP (B) (both green). Excitation transferred through the crista terminalis, pectinate muscles, and Bachmann’s bundle spreads with a velocity of 0.5 to 1 m/s (in the human heart) to the right and left atria, where Cx40 and Cx43 are most abundantly expressed,47 both exhibiting high single-channel conductances (180 pS and 115 pS, respectively),5,48 all of which is consistent with the fast spread of excitation and almost synchronous contraction of both atria. Presumably, cell-cell coupling in the atria is maintained by homotypic Cx40 and Cx43 GJ channels because Cx40 and Cx43 are highly incompatible in forming heterotypic GJs.20 The excitation wave enters the AV node through the transitional A-N zone, and its velocity of propagation gradually decreases to approximately 0.05 m/s in the central region called the compact node or N region. In a normal heart, the AV node is the sole connection between the atria and ventricles, and the delay of AV conduction is necessary for the sequential contraction of both, which is important for optimal hemodynamics. Both the relatively long refractory period of the AV-nodal cells and the dependence of the conduction velocity on the frequency of excitation limit the number of impulses within a given time period—termed the Wenckebach point—that can be transmitted to the ventricles. Thus, the AV node protects the ventricular myocardium by reducing the frequency of APs transmitted to the bundle of His during atrial fibrillation. Slow AV-nodal conduction under pathophysiologic conditions can also lead to the generation of supraventricular tachycardia involving reentry loops. In the AV-nodal cells, mCx30.2 and Cx45 are primarily expressed, both exhibiting relatively low single-channel conductances. Furthermore, Cx40 is expressed in the N region, but to a lesser extent.42
Molecular Organization, Gating, and Function of Gap Junction Channels
Connexins Expression and Oligomerization
De novo Formation of Gap Junction Channels
Voltage and Chemical Gating of Connexin-Based Channels
Hemichannels: Function and Physiologic Relevance
Connexins Expression Pattern and Propagation of Excitation in the Heart
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
