Molecular Development of the Heart: Introduction
The wide spectrum of congenital cardiovascular anomalies found from the prenatal period into adulthood has challenged clinicians and scientists for centuries.1-6 The goal of this chapter is to present a highly condensed overview of our current understanding of the normal development of the vertebrate heart and vasculature and to illustrate how this knowledge allows us to begin to define the pathogenesis of congenital cardiovascular malformations. Critical insights into the molecular regulation of vertebrate heart development and the origins of congenital heart disease have come from the investigation of species with much simpler “cardiac” structures, including the pulsatile dorsal vessel in the fly, the two-chambered hearts of zebrafish, and the three-chambered hearts of amphibians. The evolutionary addition of critical cardiac features, including cardiac valves, a high-pressured ventricle, and septation of the atria, ventricles, and outflow tract, is associated with expanded molecular complexity and redundancy.7 Although many of the mechanisms that lead to the fully septated, four-chambered vertebrate heart are interdependent, they are presented in separate sections for clarity. This chapter focuses on human development; however, numerous lower vertebrate and invertebrate animal models are accelerating our identification of the genetic and epigenetic regulation of normal and aberrant cardiovascular morphogenesis.7-12
Molecular Development of the Heart
The initial molecular programming for cardiac morphogenesis is thought to be established at the earliest stage of development with determination of the three axes of the embryo: anteroposterior, dorsoventral, and left-right. Specific genes have been identified that alter axis determination in a range of species including the mouse, frog, and chicken.13,14 After determination of the embryo axes, subpopulations of cells (somites) are programmed in a segmental body plan controlled at the molecular level by a segmentation clock and gradients of signaling molecules.15-18 In mammals, maternal gene products control the cell through the first two cell cycles; then control switches to the embryonic genome.
The process of mesoderm formation is integral to the organization of the primary axis of the embryo and the differentiation of right and left sides. At the blastodisk stage of development, there are two primitive germ layers: endoderm and ectoderm. The endoderm layer then splits into splanchnic and visceral layers, with interposed mesodermal cells (Fig. 9–1). Mesoderm is formed as ectodermal cells migrate through the primitive streak coursing adjacent to the Hensen node, and lateral plate precardiac mesodermal cells migrate to form the heart and great vessels. The Hensen node contains retinoic acid and serves as an embryonic organizer that confers information required to direct the ultimate fate of these mesodermal cells.19 At this critical phase in cell determination, exogenous retinoic acid is extremely teratogenic, with the greatest effect at the arterial pole and the least effect at the venous pole.20
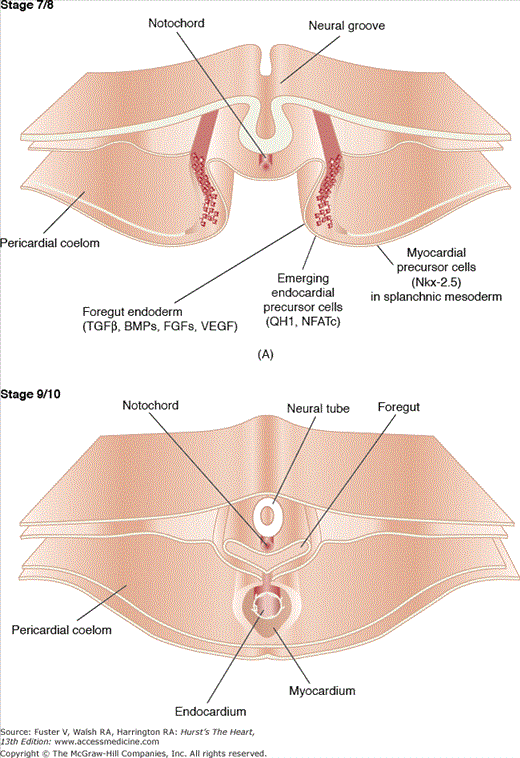
Figure 9–1.
This figure illustrates the postgastrulation morphogenetic events involved in the formation of the tubular heart. A. A quail embryo is shown at Hamburger-Hamilton (H/H) stage 7/8, demonstrating the emergence of endocardial precursor mesenchymal cells, characterized by the expression of the antigen QH1 and the transcription factor NFATc, from the splanchnic mesoderm. This mesoderm is also the source for the future myocardium, which, for instance, expresses the transcription factor Nkx-2.5. It is proposed that the formation of both endocardium and myocardium are induced by growth factors, such as transforming growth factor β (TGFβ) isoforms, bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), and vascular endothelial growth factor (VEGF). B. Subsequent to the migration and assembly of endocardial precursor mesenchymal cells during H/H stages 7 to 8, the cellular plexus coalesces to form the definitive endocardial tube enveloped by the myocardial tube. Note that the endocardium is still in close proximity to the ventral side of the foregut. Courtesy of Dr. Yukiko Sugi, Cardiovascular Developmental Biology Center, Medical University of South Carolina.
Correct laterality is a fundamental aspect of normal embryonic development, and errors in laterality occur in approximately 1 in 8000 births. Complete reversal of lateral patterning, termed situs inversus, was first described by Matthew Baillie in 1793.21 Normal cardiac laterality (situs) has the lowest risk of congenital cardiovascular malformations.22 The first grossly asymmetric feature to develop is the heart tube, which undergoes rightward looping (Fig. 9–1). However, the asymmetric expression of molecular markers occurs much earlier in development. This pattern of left-right asymmetry occurs in all vertebrate internal organs because of signaling cascades present prior to gastrulation. The current paradigm for explaining asymmetry begins with the function of surface cilia on primitive, dorsal forerunner cells in the region of the neural tube prior to gastrulation.21,23 Ciliary function at this site drives unilateral extraembryonic right to left nodal flow of fluid containing secreted morphogens.24 Abnormalities of ciliary function associated with laterality defects are termed primary ciliary dyskinesias (PCD), and up to 6% of patients with PCD can have abnormalities of cardiac situs termed heterotaxy defect. A key insight into this process came from investigating mutant mouse embryos that lack the kinesin motor protein, KIF3, and have immotile epithelial nodal cilia, randomized localization of the laterality gene lefty2, and randomized left-right determination.25 These epithelial monocilia can be subdivided into subclasses that contain or lack the motor protein left-right dynein (lrd), which is targeted by the mutant iv gene in mice that display situs inversus.26,27 The mouse inv mutant shows consistent reversal of left-to-right (L-R) patterning, which may be dependent in part on calcium-calmodulin signaling.28,29
One of the earliest morphogens regulating L-R patterning is the asymmetric phosphorylation of syndecan-2 by protein kinase C γ.30 Recent evidence suggests that the leftward movement of membrane-sheathed particles, called nodal vesicular parcels (NVPs), may result in the activation of the noncanonical hedgehog signaling pathway, an asymmetric elevation in intracellular Ca2+, and changes in gene expression.31 The critical timing of this patterning process has been shown to produce phenotypic variation from L-R reversal to randomized L-R patterning within a 1-hour window. The development of L-R asymmetry requires transcription factors that unilaterally suppress gene expression (eg, Shh induction of Nodal on the left side, which then induces the bicoid-type transcription factor Pitx2c). Targeted disruption of Pitx2c in mice results in a complex phenotype including body plan laterality defects associated with complex cardiac anomalies associated with right atrial isomerism and complex intracardiac defects.32 It is important to note that Pitx2c is also expressed later in development in the secondary heart-forming field responsible for expanding and remodeling the outflow tract; therefore, the cardiac phenotype in this mouse mutant is related to more than disrupted laterality.33 Misexpression of the normally left-sided signals Nodal, Lefty2, and Shh on the right side or ectopic application of retinoic acid results in upregulation of Nkx-3.2 contralateral to its normal expression on the left,34 whereas FGF8 inhibits Nkx-3.2 expression.35
Defining the molecular basis underlying the establishment and maintenance of cardiac muscle differentiation has presented a fundamental challenge in developmental biology and molecular genetics (Fig. 9–2), and >100 molecular factors have been identified to impact cardiovascular morphogenesis and maturation. Despite the shared expression of numerous contractile protein genes by both cardiac and skeletal striated muscles, the molecular mechanisms for cell determination, differentiation, and tissue patterning between these two tissues have some similarities but also significant differences. For example, a basic helix-loop-helix transcription factor, MyoD,36 is necessary and sufficient to commit mesodermal and nonmesodermal cell types to the skeletal muscle lineage. The MyoD family of muscle determination transcription factors are not expressed in cardiac muscle. Numerous reviews are now available that summarize recent insights in the molecular regulation of cardiovascular development in and inverbrates7,11,12 and vertebrates.14,37,38
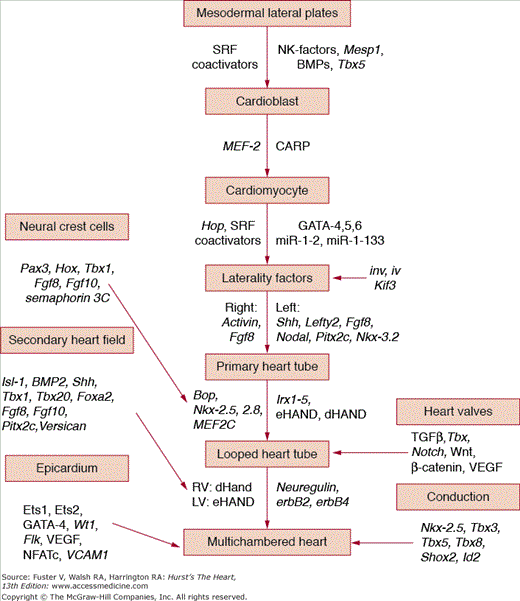
FIGURE 9–2.
Schematic cascade of some of the major genes and transcription factors proven to regulate cardiomyocyte determination, differentiation, and final phenotype. This outline is intended to display the concept of temporal and spatial regulation of a complex developmental process rather than to be comprehensive because there are now >200 genes and proteins identified to affect cardiovascular morphogenesis.
Because they are genetically more tractable, less complex organisms have been particularly useful for identifying and testing the function of genes involved in heart development. Examples include the fruit fly, Drosophila melanogaster; the zebrafish, Danio rerio; and the frog, Xenopus laevis.39,40 Homeotic genes are transcription factors that determine body structure and have in common a 60—amino acid DNA-binding domain termed the homeobox (HOX). The HOX genes are generally upregulated during early differentiation in a time-dependent sequence. HOX genes have been studied extensively in Drosophila, where they are involved in the commitment of cells to specific developmental pathways and play an important role in pattern formation.41,42 The tinman gene was the first HOX identified in the (Drosophila) heart and derives its name from the absence of heart formation when the gene is mutated.43 The vertebrate homologs termed NK HOX genes, identified in vertebrates including zebrafish, Xenopus, chickens, mice, and humans, are highly related in sequence and expression pattern44,45 and are thought to function in phylogenetically conserved cardiomyogenic pathways, although this is more difficult to define due to family member complexity and redundancy.46 The murine NK-2 HOX gene Nkx-2.5/Csx is expressed in early cardiac progenitor cells prior to cardiogenic differentiation and continues through adulthood.40Nkx-2.5 has been shown to bind to novel NKE sites, certain serum response elements of the cardiac α-actin promoter, and the NKE sites in the cardiac atrial natriuretic factor promoter.44 In addition, tinman is known to regulate NK-3/bagpipe expression in the visceral mesoderm.46 Recent evidence in Drosophila suggests that mesodermal expression of tinman requires the expression of heartless, expression of a BMP2/4-like gene decapentappleci (dpp) in adjacent ectoderm, and the expression of the dpp receptor thickvein and the Smad4 homolog medea in the mesoderm.7,47 Coexpression with GATA genes is required for tinman to drive cardiogenesis, highlighting the rule of complex and combinatorial control of gene expression in cardiac differentiation and morphogenesis. Overexpression of Nkx-2.5 in zebrafish embryo results in an enlarged heart as well as conduction defects.48 As mentioned later, patients with secundum atrial septal defect and conduction defects have now been identified to have specific mutations in the human homolog to the Nkx-2.5 gene.49
Serum response factor (SRF) is an ancient DNA-binding protein, containing a highly conserved DNA-binding/dimerization domain termed the MADS box. SRF target genes are characterized by the presence of a single copy or multiple copies of the SRF-binding consensus sequence known as the CArG box in promoters of genes that are involved in cell differentiation, growth, and contractility.50 SRF is highly expressed in heart and other mesodermally derived tissues.51 Its specificity for transcriptional activation of muscle-specific genes is provided by the myocardin family of cofactors REF. SRF-related proteins bind to the regulatory regions of >250 non—muscle- and muscle-specific genes.52 SRF frequently binds to promoter regions in combination with other factors including GATA-4 and NKX2.5. SRF-related proteins are capable of binding MEF2 sites, which can be found in the regulatory regions of both non—muscle- and muscle-specific genes.53,54 Like SRF, MEF2 factors contain an MADS box and an adjacent MEF2 box. Expression and mutagenesis studies in Drosophila have shown that D-mef2 proteins are necessary for myogenic differentiation during development55,56 and are activated by tinman.57 In the mouse embryo, MEF2 genes (of which four have been identified in vertebrate species) are highly expressed in the early heart and skeletal muscle progenitor cells prior to the induction of cardiac and skeletal muscle structural genes, implicating MEF2 as a key regulator of cardiac and skeletal muscle differentiation programs. For example, deletion of murine MEF2C interferes specifically at the point of cardiac looping. It has been shown that Nkx-2.5 transactivates the cardiac α-actin gene by binding to SRF but only after SRF has bound to DNA.58
The GATA transcription factors derive their name from their DNA-binding sequence. GATA-1, -2, and -3 are linked to hematopoiesis, and GATA-4, -5, and -6 are involved with cardiac, gut, and blood vessel formation. Each of the six GATA proteins contains a highly specific DNA-binding domain, consisting of two C4 zinc fingers that may interchangeably bind to a unique DNA sequence. GATA-4 and -6 are expressed in developmentally and lineage-specific patterns within cardiac mesoderm and gut epithelium,59,60 whereas GATA-5 expression is restricted to the epicardium. Experiments have shown that GATA-4 regulates expression of cardiac-specific genes, such as cardiac troponin C61 and αMHC.62 Several studies have demonstrated that the GATA-4 transcription factor plays an important role in regulating cardiac-specified genes and appears to be downstream to the Nkx-2.5 gene.63,64 Mice lacking the GATA-4 gene display a severe defect in cardiac tube formation. Transcriptional repressors have also been found to regulate cardiac development; and FOG-2, a zinc-finger repressor protein, functions to suppress GATA-4—mediated activation of cardiac-restricted genes.65
Bone morphogenic proteins (BMPs), members of the transforming growth factor β (TGFβ) superfamily of signaling molecules, provide critical signals for commitment to the cardiac lineage and morphogenesis.66 BMP-2 and BMP-4 can induce the cardiac regulatory factors Nkx-2.5 and GATA-4 when ectopically applied to regions of chick embryos that are not usually specified to become heart tissue.66 In mice lacking the BMP-4 gene, there was little or no mesoderm differentiation. Some mice deficient for the BMP-2 gene that lacked Nkx-2.5 expression also failed to develop beyond the early stages of looping.67-69
The first basic helix-loop-helix (bHLH) transcription factors identified in the developing vertebrate heart include dHAND and eHAND, although their expression was, unlike the MyoD family, subsequently shown to be much more widespread. These proteins share sequence homology in their bHLH regions and have temporally and spatially specific expression patterns.70 In the mouse, HAND expression coincides with that of other cardiac transcription factors under the regulation of a cardiac-specific SET domain protein, BOP, that interacts with histone deacetylases. Expression of dHAND and eHAND precedes separation of the two ventricles and is required for early chamber development; whether this reflects an ability of the factors to direct chamber specification is still under study. dHAND expression in the myocardium is maintained throughout the straight heart tube but is restricted to the conotruncus and future right ventricle (RV) as the heart tube forms a loop. eHAND expressed in the myocardium becomes rapidly restricted to the left ventricle (LV).71 Deletion of dHAND by gene targeting showed that dHAND expression is necessary for RV formation72; and although GATA-4 expression is reduced following the elimination of dHAND, the expression of cardiac-specified genes, αMHC, MLC2A, MLC2V, ANF, and Nkx–2.5, is unaffected.73 Double mutants lacking dHAND and Nkx-2.5 lack ventricular chamber development, fail to express the ventricular-specific transcription factor Irx4, and are embryo lethal.74
The expansion of unique RV and LV myocardium is under the tight regulation of molecular pathways. For example, expression of the T-box gene, Tbx5, is responsible for the specific identity of LV myocardium through interactions with both eHAND and eHAND-sensitive elements and is negatively regulated by the related Tbx20 gene that is normally expressed in the developing RV myocardium. Of note, not all mouse embryos lacking Tbx5 have a secundum atrial septal defect similar to humans with Holt-Oram syndrome, but all affected embryos have impaired ventricular function.75-79 The differential effects of changes in Tbx expression in the embryo reflect differences between the concept of heterochrony (similar genes expressed under differential temporal schemes) versus heterotopy (similar genes with differential effects based on spatial context). Mice that lack a single copy of Tbx5 have the atrial septal defect and conduction abnormality found in patients with Holt-Oram syndrome, and the zebrafish mutant, heartstrings, has a similar defect.80 Of note, Tbx5 directly regulates forelimb development via direct activation of the Fgf10 gene.81,82
Given the structural and functional complexity of the heart, it may not be surprising that no master regulator of cardiomyocyte differentiation analogous to MyoD has been identified. Tremendous progress has been made in identifying a combinatorial transcriptional cascade under the control of signaling factors that is required for cardiac specification and the temporal and spatial regulation of morphogenesis. The technologic advances that have facilitated genomic and bioinformatics approaches will enable a more comprehensive approach to the identification of the gene programs under the control of these factors and signaling pathways and pave the way for regeneration of myocardium in the mature organism.
The completion of the sequencing of the human and other genomes led to the realization that the number of genes in the most complex species, Homo sapiens, is only slightly greater than that of the earthworm Caenorhabditis elegans (25,000 vs 19,000, respectively). From this unexpected observation came the hypothesis that it is the regulation of gene expression that has the largest impact on structural and functional complexity. This regulation occurs at many levels, including transcriptional (reviewed earlier), alternative splicing of pre-mRNA, and RNA turnover and translation regulated by microRNAs (miRNAs). The miRNAs have generated considerable excitement due to their recent discovery and therapeutic potential for turning off gene expression.38,83 miRNAs are a class of approximately 700 small noncoding RNAs, typically 20 to 26 nucleotides in length, that primarily function posttranscriptionally by interacting with the 3′ untranslated region of target mRNAs in a sequence-specific manner to cause mRNA degradation and/or translational repression.84 Targeted temporal and spatial deletion of the gene DICER, which is required to process pre-miRNA into small interfering RNA, in mouse embryos has identified miRNAs to be expressed in both cardiac and vascular cell types and has shown that these small RNAs influence cardiomyocyte differentiation, proliferation, and morphogenesis. Two widely conserved miRNAs with cardiac- and skeletal muscle—specific expression are miR-1 and miR-133, which are derived from a common bicistronic transcript.38 As might be expected in heart, transcription of miR-1 and miR-133 is regulated by Mef2 and SRF.50 These two factors have opposing action, where miR-1 tends to limit the pool of proliferating ventricular myocytes via exit from the cell cycle, in part through the actions of the histone deacetylase 4 (HDAC4), which is recognized to be a transcriptional repressor of Mef2-dependent cardiac gene expression. In contrast, miR-133 stimulates cell proliferation via SRF, and targeted deletion of miR-1 and miR-2 in mice also results in cardiac hyperplasia. Further investigation into miRNAs is likely to reveal expanded roles in all facets of cardiac cell specification, differentiation, and tissue organization. Much like transcriptional control, the identification of the complete set of mRNA targets for each miRNA will be critical to advances in this field.
Numerous cell explant experiments have shown that mesoderm alone is insufficient to generate heart tissue.14 Heart formation requires temporally and spatially inductive signals from the ectoderm, the organizer, and endoderm. Endodermally expressed genes and transcription factors have been shown to influence cardiac development or to result in cardiac malformations in genetically targeted models. Conditional deletions of Shh have shown that paracrine signaling is required for morphogenesis of the secondary heart field (see Contributions of the Secondary Heart Field).85
In the human embryo, the heart begins to contract on approximately postconception day 17, indicative of functional excitation-contraction coupling. There is a highly ordered and species-specific pattern of contractile gene expression in the developing heart fields and in the early looped heart.86 For example, expression of the myosin light chain MLC2V gene is the earliest known marker of the vertebrate ventricular cardiac muscle sublineage.87,88 These functional units include the sarcomere, containing the contractile elements; the mitochondria, containing the enzymes for energy production and modulation; and the sarcolemma, including the cell envelope with specialized components of the t-tubular system linked to the sarcoplasmic reticulum (SR). In the mature myocardium, sarcomeres are organized parallel to the lines of peak systolic stress. In the embryonic myocyte, myofibrils initially appear disarrayed and become aligned in response to mechanical load as development proceeds.89 Confocal microscopic studies of the early looping heart reveal a circumferential pattern of premyofibril distribution with randomized surface focal adhesions.90,91 Despite this disordered appearance, the contraction pattern of the early embryonic heart is isotropic for only a brief period, and then the contraction and relaxation patterns of the embryonic heart become anisotropic.92,93
Multiple cis-acting regulatory elements and trans-acting factors regulate the temporal and spatial expression of contractile proteins in the developing heart across a range of species.47 At the precardiac tube stage, smooth muscle α-actin is the only isoform present. The onset of cardiac contractions is associated with a progressive increase in the expression of the cardiac form of sarcomeric actin. Smooth muscle α-actin may act as a scaffolding during assembly of the sarcomere.94-98 Much additional work is required to define the regulation of myofibrillogenesis during cardiac morphogenesis.
Mitochondria multiply concurrently with the myofibrils in the differentiating myocyte. In the mature heart, mitochondrial enzymes are the major source of high-energy phosphate necessary for contraction and likely begin this function during embryonic development. In the chick, mitochondria account for approximately 10% of myocyte volume.99 In the rat embryo, the total volume increases from 22% to 34% between days 6 and 10, and the mitochondria also change morphologically with development, becoming larger with more cristae and denser matrix.99 The myocyte mitochondrial volume fraction correlates directly with heart rate and oxygen consumption among animals.100
Maturation of the SR and apparatus for excitation-contraction coupling occurs coincident with the structural morphogenesis of the embryonic heart.101 During maturation of the heart, the resting potential increases (becomes more negative) in both birds and mammals,102 and Ca2+ influx through Ca2+ channels may play a relatively important role in transsarcolemmal Ca2+ influx in the immature heart.103,104 However, peak Ca2+ current density is actually decreased compared with that measured in mature cells.105-107 Although Ca2+ influx by way of the Na+-Ca2+ exchanger is less important for excitation-contraction coupling in mature myocardium, Na+-Ca2+ exchange plays an important role in the developing myocyte.108 In contrast to the mature myocardium, T-type Ca2+ channels also play an important role in regulating both heart rate and myocardial contractility in the embryonic myocardium.109 The molecular regulation of ion channels has been identified in the pathogenesis of adult dysrhythmias, and this process is likely to be as critical in the regulation of ion channels and embryo fate during cardiac morphogenesis.
Relaxation, an active process by which the myocardium returns to a passive, steady state after contraction, depends on the rapid removal of Ca2+ from troponin C, mediated primarily by active transport of Ca2+ back into the SR. The SR Ca2+–adenosine triphosphatase usually couples hydrolysis of adenosine triphosphate to active Ca2+ transport. The rate of SR Ca2+ uptake correlates well with the observed rate of myocardial relaxation. Regulation of SR Ca2+ pump activity is mediated by the intrinsic SR protein, phospholamban. Ca2+ is also removed from the myofilaments by extrusion across the cell membrane. In the steady state, the amount of Ca2+ removed from the myocyte equals the amount entering through the Ca2+ channels.110 Ca2+ removal from myofibers and myocardial relaxation is emerging as a critical determinant of the function of the early embryonic heart based on new evidence of the role of diastolic relaxation and suction on early heart function.111
Segmental Basis of Heart Tube Formation
Formation of the cardiac tube is a complex morphogenetic sequence. Initially, primitive, bilateral heart tubes form from lateral plate mesoderm, and each contains an inner layer of endocardium, a middle layer of cardiac jelly, and an outer layer of myocardium. The primitive heart tubes then fuse in the ventral midline to form the linear or straight heart tube.51,112 It is important to note that the primitive linear heart tube does not contain all of the cardiac segments present in the mature heart. During morphogenesis, the proximate portion of the aortic sac is incorporated into the outflow tract of the RV (along with migrating neural crest cells), and the proximate sinus venosus is incorporated into developing atria. Expansion of the RV and outflow tract requires the incorporation of cells from the anterior heart field. Thus, each segment of the mature heart arises at a unique time during embryogenesis.113 One critical aspect of this segmental assembly and maturation of the heart is that there are temporal and spatial windows that are developmentally regulated, partially explaining why morphogens such as retinoic acid can also function as potent teratogens to produce an embryo-wide spectrum of defects depending on the time in gestation of exposure. Another aspect of this segmental paradigm for molecular cardiogenesis is that cardiac morphogenesis depends on complex molecular, cellular, and biomechanical interactions between the respective segments.113
Prior to looping, the acellular space between the myocardium and endocardium in the heart is filled with a deformable extracellular matrix, the cardiac jelly, secreted by the myocardium.114 At the pretubular heart stages, the extracellular matrix contains collagen types I and IV, fibronectin, and laminin. Radioactive labeling demonstrates that proteins produced in the myocardium flow toward the endocardium and are incorporated into the basal lamina.115 The cardiac jelly has a variety of functions related to hemodynamic performance, cardiac looping, and cell migration in cardiac septation and formation of the endocardial cushion valves at the atrioventricular (AV) junction and outflow tract of the heart.
More than 100 genes have been identified in the formation of endocardial cushions, and these genes function to either stimulate or repress competitive molecular pathways.116 The protein composition of the cardiac jelly regulates endothelial differentiation via the TGFβ family of peptide growth factors.117 Some extracellular matrix proteins stimulate transdifferentiation of the endocardium in these regions by prompting endothelial cells to transform into mesenchymal cells and then migrate into the cushion matrix. Blockade of the TGFβ type I (activin receptor—like kinase), type II, and type III receptors can block this cell transformation.118-120 Smad6 negatively regulates AV cushion transformation and myocyte proliferation.121 Conversely, laminin and type IV collagen are likely stabilizing signals or markers, because these compounds are absent in the cushion regions but their presence in adjacent regions stimulates endocardial cells to maintain epithelial integrity. Periostin, the osteoblast-specific factor 2, functions to promote the differentiation of cells along a fibroblastic cell lineage toward the formation of the primary fibrous rings in the developing heart under the negative regulation of BMP-2,122 and BMP-2 and NFATc/VEGF stimulate cardiac progenitor cells to form heart valve—inducing fields.123,124 The extracellular matrix also presents a complex three-dimensional, antigenic, structural environment that directly influences cell migration, differentiation, and response to cyclic mechanical loads. The temporal and spatial secretion and remodeling of the extracellular matrix influences the fate of numerous cell populations with dramatic effects on cardiovascular phenotype and function.125-127
Expansion of the heart tube from the myoendothelial cell—lined dorsal vessel in Drosophila to the endothelial-lined larger heart walls of vertebrates relates both to the increased cell mass and structural complexity.14 Endocardial progenitor cells arise adjacent to the cardiogenic plate, in contact with foregut endoderm, begin to express flk, and stop expressing N-cadherin.128 Endocardial signals may include VEGF as well as the neuregulins via tyrosine kinases of the epidermal growth factor family. While the myocardium expresses erbB2 and erbB4, targeted ablation of neuregulin from endocardium blocks ventricular wall expansion and trabeculation.14 The endothelial cells that make up the lining of the embryonic heart are initially arranged as a single sheet. This squamouslike sheet has the morphologic features of an active tissue, including microvilli, ruffles, and intercellular openings.129 The endocardium participates in the formation of endocardial cushions at the AV junction and in the outflow tract.130 Transdifferentiation of the endocardium occurs in the endocardial cushions, where cells round up, produce pseudopodia, and migrate into the cardiac jelly.131 These cells eventually make up a portion of the fibrous skeleton of the cardiac valves. Inductive chemical signals from the myocardium contribute to the endocardial transdifferentiation and regulate the migration of the mesenchymal cells.132 In addition, hemodynamic alterations can influence the orientation of endocardial cells on the endocardial cushions133,134 and the loci of dead and dying cells in the chick and zebrafish embryos.135 Endocardial cells are also involved in patterning and remodeling of the developing outflow tract under the regulation of nuclear transcription factor NFATc1.136 Expansion of the endocardium is critical to the process of ventricular trabeculation, as discussed later in this chapter, as well as for valve formation through the expression of Msx-2, fibulin, and TGFβ1.
Following the formation of the straight heart tube, the human embryo is approximately 2 mm long and 23 days old. At the cephalic (or cranial) end of the myocardial heart tube, the nonmyocardial aortic sac can be recognized. The aortic sac is connected to the first pair of aortic arches and, later, also to the second, third, fourth, and sixth arches (the fifth pair of aortic arches does not normally develop in mammals). The caudal end of the myocardial tube receives the paired confluence of veins that lie extrapericardially and embedded in mesenchyme. In the early tubular stage, the heart hangs suspended from the ventral foregut by a dorsal mesocardium. During heart tube looping, the mid portion of the dorsal mesocardium disintegrates, leaving the heart connected at the anterior pole at the level of the aortic sac and at the posteriorly located venous pole (atria and sinus venosus). At least three distinct biomechanical mechanisms may act in combination to generate the characteristic rightward bend in the cardiac tube: locally constrained growth, active cell deformation, and the release of the prestressed dorsal mesocardium.137
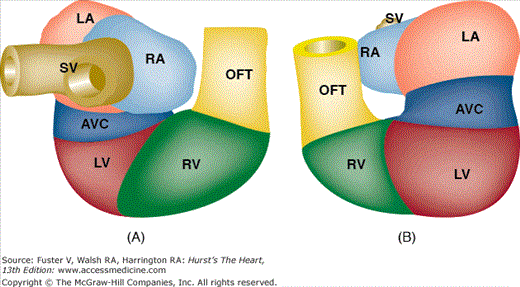
As the tubular heart continues to grow, it bends to the right and anteriorly (Fig. 9–3). This results in a compound sigmoid structure with a D-loop (dextro- or rightward) configuration. At this stage, it is easy to distinguish the sinus venosus, common atrium, AV canal, future LV and RV, and outflow segment. Internally, the developing muscular interventricular septum is recognizable, its crest characteristically expressing the molecular marker GLN2/HNK-1.138,139 It is important to note that, at this stage, all the future segments of the heart are still basically connected in series and that the common atrium connects via the AV canal exclusively to the LV, whereas the outflow tract is exclusively committed to the RV (Fig. 9–4). If cardiac morphogenesis fails to progress beyond this state, the cardiac anatomy will include a double-inlet LV and a double-outlet RV (DORV), as discussed later in this chapter.
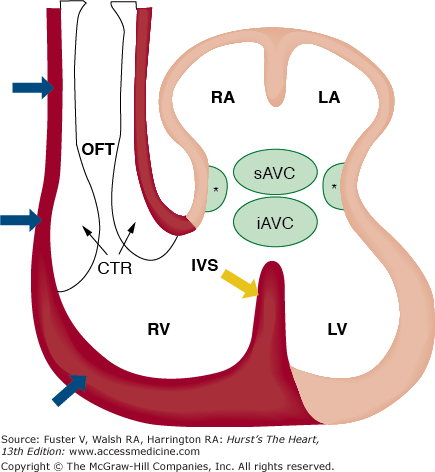
FIGURE 9–3.
Schematic representation of the contribution of the anterior/secondary heart fields to the developing heart. This illustration shows that the myocardial structures of the left atrium (LA), right atrium (RA), and left ventricle (LV) are derived from cells in the lateral plate mesoderm identified as the primary heart fields (beige). In contrast, the endothelial and myocardial tissues (red) of the outflow tract, right ventricle (RV), and portions of the interventricular septum (red) develop as derivatives of cells from the anterior/secondary heart fields. Asterisks (*), lateral atrioventricular cushions; CTR, conotruncal ridge; IVS, interventricular septum; iAVC, inferior atrioventricular cushion; OFT, outflow tract; sAVC, superior atrioventricular cushions. Adapted from Verz et al.159
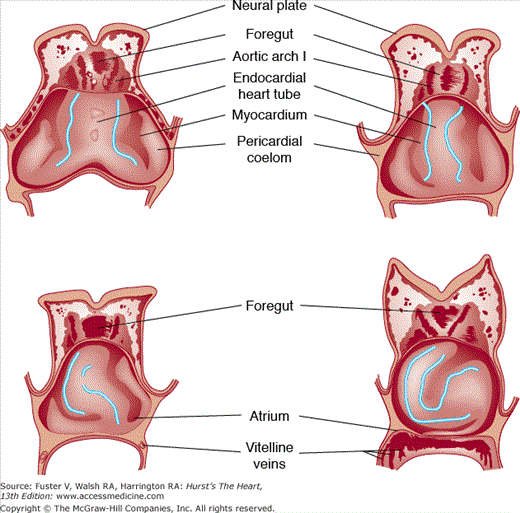
FIGURE 9–4.
Schematic ventral dissections of human embryos of different ages, showing formation of the heart loop. Adapted from Davis CL. Development of the human heart from its first appearance to the state found in embryos of 20 paired somites. Contrib Embryol. 1927;19:245. Reproduced with permission from the Carnegie Institution of Washington, DC.
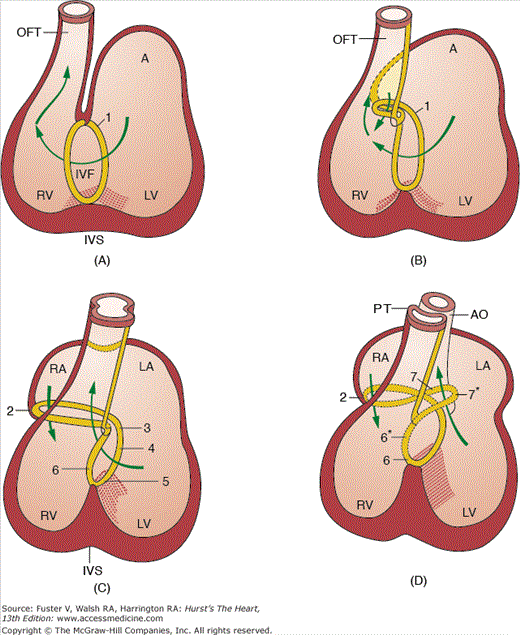
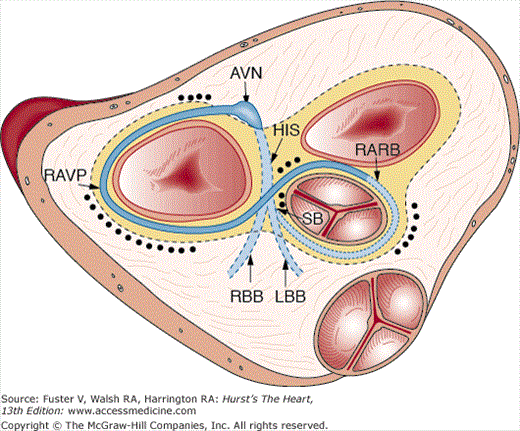
The transition from a tubular heart, in which the future segments are arranged in series (atrium to LV to RV to outflow tract), into a four-chambered heart, in which the definitive chambers are arranged in parallel separated by septa and valves, raises two important questions. The first is how the right atrium becomes connected to the RV, and the second is how the LV gains access to the aortic portion of the outflow tract. The remodeling of the so-called inner curvature of the looping heart tube plays an important role in this process and involves a rightward expansion of the AV canal and a concomitant leftward shift of the aorta.140 Immunohistochemical studies have demonstrated that this remodeling is intimately related to the development of the so-called primary ring (Fig. 9–5).140 In the postnatal human heart, derivatives of the primary ring are found in the AV conduction system, in the right AV junction (the right AV ring), and behind the aorta (the retroaortic root branch) (Fig. 9–6).139
FIGURE 9–5.
Schematic representation of the tubular heart during looping. A. Posterior view of the heart. B. Anterior view of the heart. Note that at this stage (approximately 4 weeks of development in the human), all the segments are more or less arranged in series. From inflow to outflow: SV, sinus venosus; RA, right atrium; LA, left atrium; AVC, atrioventricular canal; LV, left ventricle; RV, right ventricle; OFT, outflow tract.
FIGURE 9–6.
Schematic representation of the location of the primary ring (characterized by the expression of the antigen G1N2) in different stages of human development. The drawings illustrate the development of the conduction system as a derivative of the primary ring but also show that the changes in the topography of the ring tissue is reflecting the rightward expansion of the atrioventricular canal and the leftward shift (wedging) of the developing aorta. 1, primary ring; 2, right atrioventricular ring; 3, atrioventricular nodal area; 4, penetrating His bundle; 6, septal branch; 7, retroaortic branch. Those areas marked with an asterisk have lost their expression. The Carnegie stages of development presented in parts A to D are as follows: A, stage 14; B, stage 15; C, stage 17; and D, stages 18 and 19. A, atrium; AO, aorta; LV, left ventricle; IVF, interventricular foramen; IVS, interventricular septum; LA, left atrium; OFT, outflow tract; PT, pulmonary trunk; RA, right atrium; RV, right ventricle. Adapted from Icardo and Fernandez-Teran.175
If the primitive heart tube loops to the left and anterior (L loop) rather than to the right and anterior, most of the structures adjacent to and including ventricular segments of the heart tube (the AV valves, ventricles, and arterial roots) will develop in an inverted position. Subsequently, the right atrium is connected via a morphologic mitral valve to a morphologic LV, and the left atrium is connected via a morphologic tricuspid valve to a morphologic RV. Within the aortic sac, the aorticopulmonary septum develops in a normal fashion. However, as partitioning of the inverted conotruncus (outflow tract) takes place in mirror image, the end result is L-transposition of the great arteries, with the aorta arising anteriorly from a left-sided, morphologically right (systemic) ventricle and the pulmonary trunk (PT) arising posteriorly from a right-sided, morphologically left (venous) ventricle. Because systemic and pulmonary venous return are still routed to the pulmonary and systemic arterial circulations, respectively, this anomaly is commonly referred to as corrected transposition.
This anomaly is caused by a failure in the leftward repositioning of the aortic portion of the outflow tract, resulting in persistence of the more primitive embryonic morphology in which the entire outflow tract originates from the RV. One morphologic hallmark of the failure of completion of the leftward shift of the aorta is the presence of myocardial tissue between the left AV valve and the aorta (mitral-aortic separation). This anomaly is found following a wide range of hemodynamic, metabolic, and genetic insults to the embryo, suggesting that the phenotype of DORV may be a final common expression of a range of primary abnormalities that result in persistence of the embryonic configuration.141
The neural crest functions as the origin for migrating pluripotent cell populations with broad developmental fates. The “cardiac” neural crest is an important migratory cell population contributing to cardiovascular morphogenesis.142,143 The cardiac neural crest arises from the dorsal margin of the neural tube prior to fusion and migrates ventrally to form the autonomic ganglia, melanocytes, and Schwann cells. The crest cells move in waves through the branchial arches during the first 4 weeks of human development. The eventual fate of the neural crest cells is likely determined long before the initial phenotypic expression of a heart tube by activation of cellular gradients of HOX genes and other morphoregulating factors.144 The cranial neural crest region defines a developmental field that includes the heart, hind brain, face, and branchial arch derivatives.
Experimental disruption of cranial neural crest produces a spectrum of cardiac abnormalities. In a series of elegant ablation and chick/quail chimera studies, Hutson and Kirby143 defined the region of cardiac neural crest that is integral to the septation of the conotruncal region of the heart and branchial arch derivatives including facial abnormalities, thymus, parathyroid, and autonomic derivatives. These neural crest cells migrate to specific sites from the neural crest through the pharyngeal arches to the developing heart and carry information critical both to normal CV morphogenesis and function.143
Several genes are recognized as key factors required for the proper migration and differentiation of the cardiac neural crest. Transgenic mouse mutants lacking the endothelin (ET) peptide ET-1 or ET receptors ET-A and ET-B express abnormal cardiac neural crest phenotypes including abnormal pharyngeal arches and outflow tract septation similar to the avian neural crest ablation model.145 The splotch mutant mouse contains a mutated Pax3 gene, and homozygote splotch mutants have a complete neural crest ablation phenotype, including persistent truncus arteriosus and aortic arch anomalies146 similar to the cardiovascular phenotype of neural crest ablation in the chick embryo. HOX gene abnormalities are also associated with defects in the derivatives of cranial neural crest.147 A transgenic murine model of Hox-1.1 overexpression has neural crest ectomesenchymal tissue abnormalities including cleft palate, nonfused pinnae, and open eyes. Hox-1.5—deficient mice have features of DiGeorge syndrome.147 In humans, DiGeorge syndrome, velocardiofacial syndrome, and conotruncal anomaly face syndrome are associated with chromosomal deletions in the 22q11 region on the long arm of chromosome 22.148 Recent studies have indicated a number of candidate factors involved in the pathogenesis of these syndromes, including Tbx1.149 Interestingly, Tbx1 is expressed in the pharyngeal endoderm but not in migrating neural crest cells, so this gene regulates morphogenesis via secreted paracrine signals, including fibroblast growth factor (FGF) 8 and FGF10, to determine neural crest cell fate.150 Another secreted factor, semaphorin 3C, and the associated coreceptor, plexin A2, are also required for normal migration of pharyngeal and cardiac neural crest cells.151,152 In addition to genetic mechanisms, exogenous dosing of retinoic acid acts as a potent teratogen in humans and produces a syndrome involving all the derivatives of the cranial neural crest.153,154
Although it is clear that the ventricular myocardium expands by a process of clonal expansion, the recruitment of mesenchymal cells into a cardiomyocyte lineage from the secondary heart field is also be required for normal formation of the right ventricular outflow tract and great vessels (Fig. 9–7).37,155-159 These cells originate medial and caudal to the cardiac crescent and are displaced anterior and dorsal to the linear heart tube during cardiac morphogenesis.37 The regulation of myocyte specification and differentiation in the anterior heart-forming region likely involves cell adhesion molecules, including N-cadherin, extracellular proteases, and morphogenetic signals from the TGFβ and FGF families of growth and differentiation factors.160,161 Recent data suggest major roles for the T-box transcription factor Tbx1 and the growth factors Fgf8 and Fgf10 in regulating the fate of these cells in contributing to the aortic arches and expanding right ventricular outflow tract.162-167 The transcription factor Tbx1 is expressed in the pharyngeal arch under the regulation of Shh and the forkhead transcription factor Foxa2, and Tbx1-expressing cells are recruited from the anterior heart-forming region to expand the developing outflow tract.168 Remodeling the outflow tract involves programmed cell death likely regulated by mechanical load and by tissue oxygen content.169,170 These cells express the LIM-homeodomain protein Islet-1 very early, and this expression is required for downstream gene activation and secondary heart field formation.171,172 Isl1 regulates Mef2c gene expression with the additional requirement for GATA factors. Control of cardiac cell proliferation is also a central feature of secondary heart field development, and secreted Wnt glycoproteins and the Wnt/β-catenin signaling pathways are clearly involved in this process.173 Both Fgf8 and Fgf10 are downstream targets of Wnt signaling and function in a complementary fashion. BMP signaling is also required for secondary heart field expansion, perhaps due to the requirement of BMP signaling for Nkx-2.5 expression.
FIGURE 9–7.
Schematic representation of the localization of remnants of the primary ring in the neonatal human heart. The ring is projected on a superior view of the aortic mitral fibrous unit of the heart. The black dots indicate the areas in which remnants of the ring are detected in a series of neonatal hearts as described in Wessels et al.191 AVN, atrioventricular node; His, bundle of His; LBB, left bundle branch; RARB, retroaortic root branch; RAVR, right atrioventricular ring; RBB, right bundle branch; SB, septal branch.
Another important feature of the addition of cardiomyocytes derived from the secondary heart field is the unique electrophysiologic properties of these cells versus cells derived from the primary heart tube and the role this heterogeneity may play with respect to cardiac arrhythmias.174 Interactions between migrating neural crest cells that form the aortopulmonary septum and these secondary heart field—derived cells likely increase this organizational complexity. The outflow track has slow conducting properties until incorporated into the ventricles, and subepicardial myocytes have distinct molecular and electrophysiologic signatures.174 It is worth noting that one of the frequent ventricular tachycardias noted following congenital heart surgery originates in the outflow track, even in patients in whom the surgical interventions have been remote to this region.
The processes of primary myocardial trabeculation, expansion of secondary and tertiary myocardial trabeculae, and myocardial compaction are critical to the structural maturation of the ventricular chambers. This process results in the transformation of the smooth-walled endocardial lining into the complex three-dimensional structure of the right and left ventricular myocardium. Rapid cell division and interposition of endothelial cells along the right and left ventrolateral borders of the endocardial tube are associated with a rapid resorption of cardiac jelly, resulting in myocardial ridges and trabeculae lined with single layers of endocardial cells.175 The initial number and orientation of the myocardial ridges differ between species.176 In general, myocardial trabeculation begins at the ventricular outer curvature (future apex) and then extends proximally and distally. The intersection between the outer, compact myocardium and the base of the trabeculae is likely a site of peak wall stress, and myocyte division is most active at this site.177 Retroviral marker studies have also shown that ventricular myocardial growth is associated with a transmural distribution of clonally related myocardial cells extending from the epi- to endocardium.178,179 Of note, these cells reside in muscle bundles that are oriented at an angle to the longitudinal axis of the heart, consistent with the adult myocardial architecture, which results in efficient twist and contraction.89 However, the mechanisms that regulate clonal myocardial expansion and compaction remain undefined.
The filling capacity of the heart is increased by the added intertrabecular spaces (Fig. 9–8). The trabeculating embryonic heart can now be divided into primitive RV and LV, because there are distinct morphologic differences between the trabecular architecture of the developing ventricular chambers. The developing LV is trabeculated along most of its greater curvature, whereas the developing RV has a significant portion of the greater curvature that is smooth walled.180,181
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree