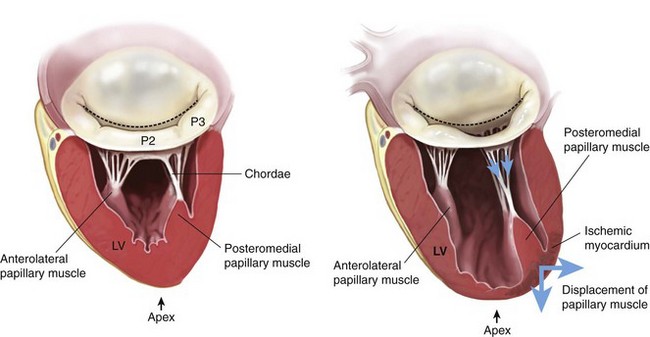
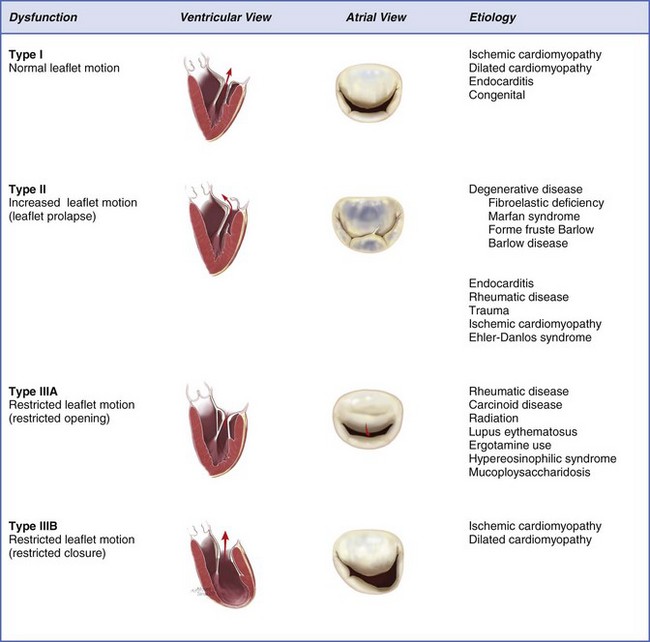
Chapter 21 The normal mitral valve is located in the left atrioventricular groove and allows unidirectional flow of oxygenated blood from the left atrium into the left ventricle in a near frictionless fashion during diastole. 1 The valve is a complex three-dimensional assembly of independent anatomical components: the annulus, the leaflets and commissures, the chordae tendineae, the papillary muscles, and the left ventricle (see Chapter 2). During systole, a coordinated interaction of these anatomic components seals the valve against left ventricular (LV) pressure. Although even a normal competent valve may present a physiologically trivial amount of reversed flow into the left atrium, more than a trace of mitral regurgitation (MR) is considered pathologic. 2 According to the natural history of the disease, although mild to moderate MR might be well tolerated indefinitely, severe MR eventually leads to LV remodeling, heart failure, and ultimately death (see Chapter 20). 3 In this context, the natural history of MR depends intimately on its etiology, the severity of LV volume overload as well as its contractile performance, and the appearance of overlapping clinical conditions secondary to reversal of flow, such as atrial fibrillation and pulmonary hypertension. 4 Primary degenerative mitral valve disease is the most prevalent cause of isolated severe MR in the United States. 5 Distinct pathologic features of the disease include mitral valve billowing (intact valve coaptation) and prolapse (deficient valve coaptation) due to myxomatous degeneration of the mitral leaflets, chordal elongation or rupture, or papillary muscle elongation or rupture. 6 In the setting of severe MR, even in the absence of symptoms, mitral valve repair is currently the gold standard procedure for patients who require surgery for degenerative MR. 7 In this particular subset of patients, mitral valve repair has become feasible and safe, and repair techniques have been proven to have an excellent durability, especially when performed in high-volume institutions. 8 In this regard, the latest guidelines for the management of patients with valvular heart disease suggest targeted referral to “reference centers” with experienced surgeons to ensure a repair rate greater than 90% and a mortality rate equal to or less than 1%. 9 Although these new standards have triggered a more liberal referral of asymptomatic patients, mitral valve repair is still underutilized in the United States. 10 Simple lesions such as posterior leaflet prolapse are associated with very high mitral valve repair rates in many centers,11,12 but the overall repair rate for more complex scenarios, as defined by leaflet involvement (e.g., isolated anterior leaflet or bileaflet prolapse), lesion complexity (e.g., significant annular calcification, significant excess tissue), or patient comorbidities (e.g., older age, reoperations), remains uncertain and seems to be well below guidelines’ recommendations.13,14 A data analysis from the Society of Thoracic Surgeons (STS) observed an average mitral valve repair rate of only 70%. 15 The following section is a review of the surgical anatomy of the mitral valve as well as an updated summary of causes, consequences, and surgical treatment of mitral valve disease. We focus on mitral valve repair for primary forms of MR such as annular dilation and mitral valve prolapse, controversies on mitral valve repair versus mitral valve replacement for patients with secondary MR (see next section), and, finally, on mitral valve replacement for those etiologies of MR not as amenable to mitral valve repair, such as rheumatic mitral valve disease. As previously noted, the normal mitral valve is a dynamic complex of independent anatomic structures. The presence of abnormalities in any of these components (lesions) may cause the alteration in closure (dysfunction) against LV pressure and, consequently, MR. Structural abnormalities of the mitral valve are referred to as primary mitral valve disease, whereas valve dysfunction secondary to perturbations in LV geometry is termed ischemic MR in ischemic cardiomyopathy and functional MR in dilated cardiomyopathy (see Chapter 19). The mitral annulus is a discontinuous fibromuscular D-shaped ring located in the left atrioventricular groove (between the left ventricle and the left atrium) that serves as an anchor and hinge point for the mitral valve leaflets. The mitral annulus might be subjectively divided into anterior and posterior segments according to the attachments of the anterior and posterior mitral leaflets. In addition, it can also be segmented by location into septal and lateral components. The anterior part of the mitral annulus is in continuity with the fibrous skeleton of the heart, and is limited by the right and left fibrous trigones and the aortic mitral curtain (continuity at the level of the left and noncoronary aortic valve cusps). In contrast, the posterior part of the mitral annulus lacks a fibrous skeleton and is more prone to dilation and calcification. 16 The resultant changes in annular dimensions lead to a more circular annulus, compared with its normal “kidney bean” shape, which in turn compromises the coaptation of the mitral leaflets. The normal mitral annulus also has a three-dimensional saddle shape with two lower points at the level of both trigones and one peak at the midpoint of the anterior leaflet. This peak point is always above the midpoint of the posterior leaflet, allowing bulging during systole to accommodate the aortic root and optimize stress distribution over both leaflets. The overall circumference of the annulus may decrease by as much as 20% during systole (less eccentricity), promoting central leaflet coaptation. 17 Reduction in annular size begins with atrial contraction and reaches its maximum halfway through the systolic cycle. The mitral valve has two leaflets (anterior and posterior) with similar surface areas and thicknesses (≈1 mm) but significantly different shapes. The anterior leaflet is taller and has a shorter base than the posterior leaflet, extends vertically, and is anchored to one third of the annular circumference between the right and left fibrous trigones. 18 The posterior leaflet is broader based, has a shorter height than the anterior leaflet, lies transverse to the mitral valve orifice, and, together with the commissures, is fixed to the remaining two thirds of the annulus. The posterior leaflet is closely related to the LV wall base, the point of greatest systolic stress. It is important to emphasize that the different orientation of the two leaflets ensures a competent closure line of the mitral valve during systole, located in the posterior one third of the valve orifice, which naturally prevents systolic anterior motion. 19 Additionally, both leaflets present two zones from its base to the free border or margin: the atrial or membranous zone (smooth and translucent) and the coaptation zone (rough, nodular, and thicker due to the attachment and fusion of chordae tendineae). As a surgical reference, the leaflets of the mitral valve can be “segmented” by location of the clefts or indentations in the posterior leaflet. If one counts both commissures as individual segments, a total of eight segments can be identified. Unlike the anterior leaflet, the posterior leaflet has two clefts in its free margin that allow full opening during LV filling and that, in turn, demarcate three segments or scallops. The middle scallop of the posterior leaflet is designated P2 and adjacent medial and lateral scallops are designated as P1 and P3, respectively. The corresponding areas of the anterior leaflet are designated by opposition to the segments in the posterior leaflet as A1, A2, and A3 ( Figure 21-1). FIGURE 21-1 Anatomy of the mitral valve and mitral apparatus. The chordate tendineae are filament-like structures of connective fibrous tissue that join the LV surface and free border of the mitral leaflets to the papillary muscles and, by default, the posterior wall of the left ventricle. They create a suspension system that allows full opening of the leaflets during diastole and prevents the excursion of the leaflets above the annular plane during systole. 20 A total of about 25 primary chordae begin in the papillary muscles and progressively subdivide to insert into the leaflets. Chordae tendineae are classified according to their insertion point between the free border and the base of the mitral leaflets. Primary or marginal chordae attach along the margin (every 3 to 5 mm) of the leaflets and are critical to prevent leaflet prolapse and to align the rough zone of the anterior and posterior leaflets during systole. Secondary or intermediate chordae, which are inserted in the ventricular side of the body of the leaflets, relieve excess tension during systole. 21 Tertiary or basal chordae are only found on the posterior leaflet and connect its base and the posterior annulus to the papillary muscles, providing additional linkage to the ventricle. The mitral valve leaflets are attached by the chordae tendineae to the papillary muscles, which are considered an extension of the left ventricle. The papillary muscles vary in the number of heads and exact position in the ventricle, but generally two organized groups can be identified. Each papillary muscle is designated according to the relationship to the valve commissures, and each provides a fan chord to its corresponding commissure as well as to both the anterior and posterior leaflets. The anterior papillary muscle has a single body, is larger, and is irrigated by the first obtuse marginal branch of the circumflex artery and the first diagonal branch of the anterior descending artery. The posterior papillary muscle has two bodies, is smaller, and is irrigated only by the posterior descending artery, a branch of the right coronary artery, in 90% of cases and by the circumflex artery in the other 10%. This arrangement explains the relative vulnerability of the posterior papillary muscle to ischemia, and subsequent involvement in localized remodeling in the setting of ischemic MR. 22 The left ventricle supports the entire mitral apparatus, owing to its continuation with the papillary muscles, and thus LV dimensional changes in the setting of volume overload and remodeling, whether ischemic or not, can lead to leaflet tethering and MR. 23 MR is defined as the existence of blood flow in systole from the left ventricle into the left atrium. The presence of minimal structural lesions might cause MR by reducing mitral leaflet coaptation. Therefore, the exhaustive interrogation (identification, localization, and magnitude) for mitral lesions is essential to determining the chances of successful valve repair and proceeding with a tailored therapeutic plan for each patient. Three decades ago, Carpentier described a systematic analytic approach to patients with MR known as the “pathophysiologic triad of MR.” 24 The triad emphasizes the importance of distinguishing between the medical conditions causing MR (etiology), identifying the resulting lesions, and finally how these lesions affect leaflet motion (dysfunction). Nowadays, besides promoting mutual understanding among surgeons and specialists in cardiac imaging, the triad also represents an organized and very consistent way to elucidate the most appropriate techniques to achieve a successful repair. The differentiation of valve dysfunctions (I, II, and III) is based on the position of the leaflet margins with respect to the mitral annular plane ( Figure 21-2). Type I dysfunction implies normal leaflet motion, and the most common cause of significant MR is the perforation of one of the leaflets (e.g., endocarditis) or severe annular dilation with a central regurgitant jet (e.g., primary atrial fibrillation) ( Figure 21-3A). Type II dysfunction denotes excess leaflet motion generally secondary to chordal elongation or rupture or to myxomatous degeneration of the leaflets (regurgitant jet directed to the opposite site of the prolapsing leaflet). Type III dysfunction designates restricted leaflet motion and results typically from retraction of the subvalvular apparatus (IIIA, rheumatic valve disease or other inflammatory scenarios that lead to scarring and calcification) or from papillary muscle displacement (leaflet tethering) due to LV remodeling or dilation (IIIB, ischemic or dilated cardiomyopathy). FIGURE 21-2 Pathophysiologic triad approach to mitral valve regurgitation, composed of leaflet dysfunction, ventricular and atrial views, and etiology. FIGURE 21-3 Valve lesions in mitral valve regurgitation. Worldwide, rheumatic disease remains the most common cause of MR, but it has ceased to be the leading cause in developed countries. 25 Ischemic disease, currently responsible for 20% of cases of MR, may lose importance as a result of the ever more aggressive percutaneous treatment of coronary artery disease. Thus, degenerative disease is today the most frequent cause of MR in western countries. 26 Degenerative mitral valve disease is characterized by a wide spectrum of lesions, 27 varying from a simple chordal rupture leading to prolapse of an isolated segment (frequently P2) in an otherwise normal valve, to multiple segment prolapse of both leaflets in a valve with significant excess tissue ( Figure 21-3A and B). 28 This range of lesions gives rise to two opposing entities: fibroelastic deficiency and Barlow disease. 29 Fibroelastic deficiency occurs in older patients (generally more than 60-years-old) with a short history of severe holosystolic murmur. As the term fibroelastic implies, this disease is a condition associated with a deficit of the protein fibrillin that often leads to weakening, elongation, and ultimately rupture of chordae tendineae. 30 Chordal rupture of P2 is considered the most common lesion in patients with fibroelastic deficiency. The mitral leaflets are usually thin and translucent, although the prolapsing scallop might have a myxomatous aspect if the disease has been present for a long time. Distinguishing fibroelastic deficiency from other entities within the spectrum of degenerative mitral valve disease requires an exhaustive analysis of those segments immediately contiguous to the prolapsing one, which are generally normal in size, height, and tissue properties. Finally, the annular size in patients with this condition is often less than 32 mm. In contrast, at the opposite end of the spectrum of degenerative disease is Barlow disease. 31 Affected patients are younger, usually less than 60 years old, and present with a long history of holosystolic murmur that has been monitored by the referring cardiologist for many years. In this context, patients with Barlow disease have a more diffuse and complex redundancy of the leaflets. The most common lesions are excess leaflet tissue, leaflet thickening and distension, with diffuse chordal elongation, thickening, and/or rupture. 32 In these patients, the annular size exceeds 36 mm, and it is not uncommon to find varying degrees of annular calcification (often involving the anterior papillary muscle) as well as fibrosis of the subvalvular apparatus 33 ( Figure 21-4). FIGURE 21-4 Most typical clinical and surgical differences between fibroelastic deficiency and Barlow disease. Rheumatic disease is still the main cause of mitral disease in underdeveloped or developing countries (see Chapter 17). A systemic exudative inflammatory reaction involves the connective tissue of skin, joints, and heart. 25 Cardiac involvement has been described as a pancarditis with characteristic implication of the left-sided valves. Severe edema and cellular infiltration (severe leaflet thickening extending toward the commissures) is followed by the formation of rheumatic nodules along the free borders of the leaflets. In addition, all the components of the subvalvular apparatus are also affected, leading to chordal thickening and retraction and chordal and commissural fusion. The annulus then dilates in a very asymmetric fashion predominantly along the P3 segment (see Figure 21-3C). It is important to highlight that the anterior leaflet is generally less affected than the posterior leaflet, which is often retracted. Owing to the complexity of lesions, rheumatic mitral disease is not as amenable to valve repair. 34 Ischemic MR is a consequence of myocardial ischemia and remodeling. In this context, ischemic MR can manifest acutely after papillary muscle rupture (primary) or secondary to LV remodeling and apical and inferior displacement of the papillary muscles. 35 In the presence of ischemic MR, the mitral leaflets are tethered, and their coaptation point is below the mitral annulus (see Figure 21-3D). When restricted leaflet movement occurs principally in systole, the pattern is asymmetric and is mainly observed in patients with posterior infarction and posterior leaflet restriction (eccentric regurgitant jet) 36 ( Figure 21-5). In contrast, in patients with dilated cardiomyopathy or anterior and posterior infarctions, both leaflets have a restrictive deficit, giving rise to a symmetric pattern (central jet). 37 In order to understand ischemic MR and its surgical approach, it is critical to understand the mechanism (secondary classification) and the dynamics of the disease (possible progression). 38 The analysis of the mechanism of MR, which is even more critical, answers prognostic questions such as How tethered and angulated are the leaflets? Is there a pseudoprolapse? Is the regurgitation jet eccentric or central? What are the ventricular dimensions? How reversible is the ischemic insult? 39 MR predisposes the left ventricle to a volume overload in order to compensate for the volume lost to regurgitation. Although mild to moderate MR might be well tolerated for long periods, severe MR is fatal at a determined stage. Severe MR can be mainly divided into three clinical stages—acute, chronic compensated, and chronic decompensated—each of which requires different management and has different surgical triggers. In this regard, severe MR is a mechanical problem with surgery as the only definitive solution, either mitral valve repair or mitral valve replacement. Although the lack of randomized trials comparing mitral valve repair with replacement has led to controversy, particularly in the setting of secondary MR, repair is favored over replacement for multiple reasons, especially in patients with degenerative mitral valve disease 40 ( Figure 21-6). The reasons include a likely lower perioperative risk and improved event-free survival in the majority of operated patients, freedom from the various complications of prosthetic heart valves ( Figure 21-7), and better postoperative LV function. 41 FIGURE 21-6 Probability of survival (death from any cause) among patients having mitral valve repair versus replacement for posterior leaflet prolapse (A) and bileaflet prolapse (B). (From Suri RM, Schaff HV, Dearani JA, et al: Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006;82:819-826.) FIGURE 21-7 A, Incidence of first ischemic stroke and B, thromboembolism after surgery for mitral regurgitation. (From Russo A, Grigioni F, Avierinos JF, et al: Thromboembolic complications after surgical correction of mitral regurgitation incidence, predictors, and clinical implications. J Am Coll Cardiol 2008;51:1203-1211.) Several surgical approaches for access to the mitral valve have been described. Although the earliest mitral valve procedures were performed through a right thoracotomy, the mitral valve has traditionally been exposed through a median sternotomy. Nowadays, median sternotomy remains as the gold standard and it is still the most popular approach. 42 Central cannulation and direct aortic clamping enable mitral surgery with generous exposure and excellent results. Some groups have significantly transformed the incision to a lower hemisternotomy, thus limiting the length of the incision to 7 to 9 cm. However, in an effort to reduce invasiveness and the potential operative morbidity, cardiac surgeons have adopted nonsternotomy, also known as video-assisted approaches, including right thoracotomy and robotic surgery43–45 ( Table 21-1). Although the safety and efficacy of minimally invasive cardiac surgery have been established in several high-volume specialized centers, potential issues have been raised, including a higher incidence of certain complications, such as postoperative stroke. 46 Moreover, one of the issues implies the compromise of repair rates because minimally invasive cardiac surgery has been shown to be most predictably effective when utilized in simple pathology rather than in complex valve surgery. Finally, it is important to emphasize that to date there have been no clearly demonstrable clinical benefits of minimally invasive access other than cosmetic advantages. TABLE 21-1 Technical Variations and Limitations According to Surgical Approach
Mitral Valve Repair and Replacement
Surgical Anatomy of the Mitral Valve
Mitral Annulus
Mitral Leaflets and Commissures
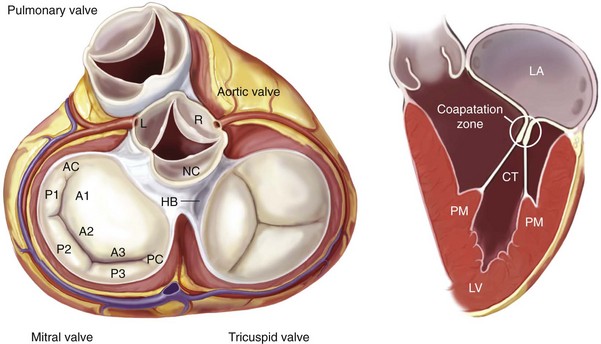
Left, Anatomic view of the cardiac valves in systole with the left and right atrium cropped away and the great vessels transected. The mitral valve apparatus consists of the mitral leaflets, mitral annulus, chordae tendineae, the papillary muscles, and the left ventricle. Right, Normal function of the mitral apparatus brings both leaflets together in systole and creates the coaptation zone. AC, Anterior commissure; A1, A2, A3, segments of the anterior leaflet; CT, chordae tendineae; HB, His bundle; L, left coronary cusp; LA, left atrium; NC, noncoronary cusp; P1, P2, P3, segments of the posterior leaflet; PC, posterior commissure; PM, papillary muscle; LV, left ventricle; R, right coronary cusp.
The Chordae Tendineae
Papillary Muscles and the Left Ventricle
The Pathophysiologic Triad of Mitral Valve Regurgitation
Dysfunction
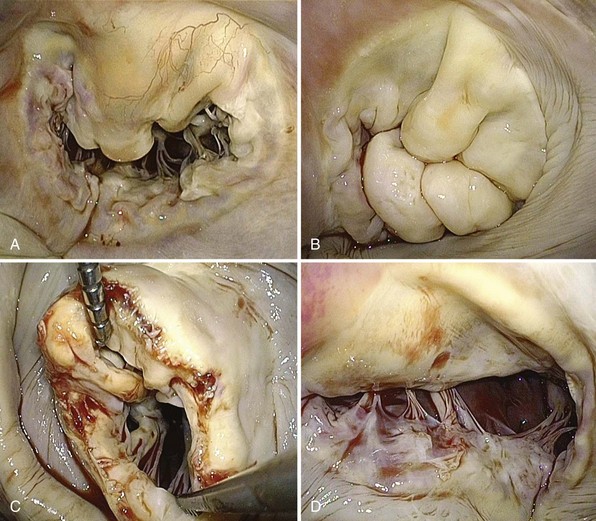
A, Severe annular dilation leading to type I dysfunction. B, Severe myxomatous changes with redundant, thick, and bulky segments in a patient with Barlow disease and type II dysfunction. C, Rheumatic mitral valve disease with classic “fish-mouth” appearance and type IIIA dysfunction. D, Ischemic mitral valve disease due to severe tethering of P3 leading to type IIIB dysfunction.
Etiology and Lesions
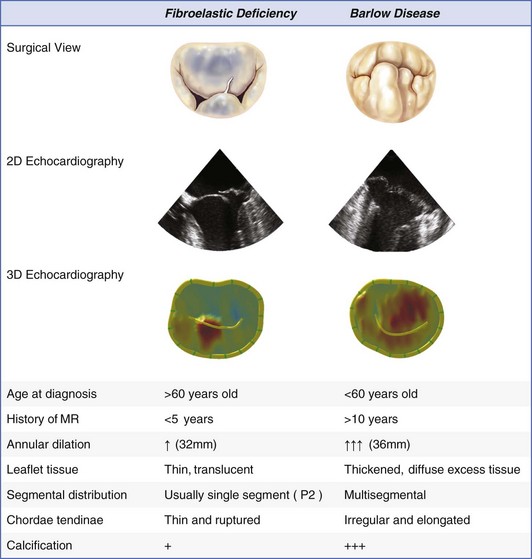
2D, two-dimensional; 3D, three-dimensional; MR, mitral regurgitation; P2, middle segment of posterior leaflet.
Mitral Valve Surgery
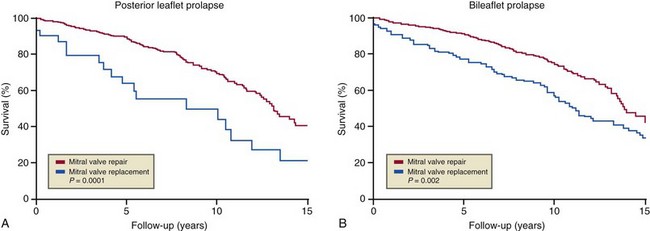
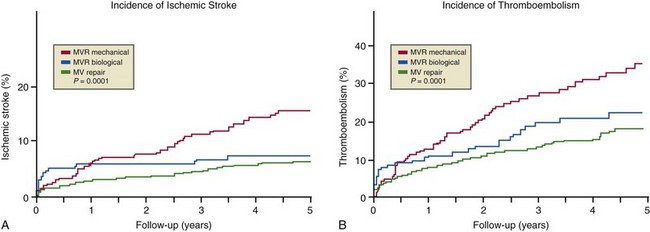
Surgical Approach
SURGICAL APPROACH
STERNOTOMY
VIDEO-ASSISTED APPROACHES
Incisions
Median sternotomy or lower hemisternotomy (7 to 9 cm)
Several 2- to 3-cm intercostal ports and an additional 4- to 6-cm working port for nonrobotic approaches
Arterial perfusion
Antegrade (aorta)
Retrograde (right femoral artery)
Venous drainage
Central
Peripheral
Aortic clamping
Clamp, site detected by direct palpation and verified by epi-aortic ultrasound
Endoscopic clamp or balloon
Myocardial protection
Direct
Indirect by rapid injection
Visualization of field
Direct and wide for all members of the team
Limited to the endoscopic device
Annuloplasty devices
As desired
Flexible posterior bands
Annuloplasty sutures
Braided polyester
Braided polyester, running polypropylene, nitinol clips
Repair techniques
As desired
Predilection for nonresectional techniques
Ventilation
Two-lung ventilation
Single-lung ventilation, potential rib spreading ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mitral Valve Repair and Replacement
Only gold members can continue reading. Log In or Register to continue