Mitral Regurgitation
Michael H. Crawford, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Dyspnea or orthopnea.
Dyspnea or orthopnea.
![]() Characteristic apical systolic murmur.
Characteristic apical systolic murmur.
![]() Color-flow Doppler echocardiographic evidence of systolic regurgitation into the left atrium.
Color-flow Doppler echocardiographic evidence of systolic regurgitation into the left atrium.
 General Considerations
General Considerations
The mitral apparatus consists of the left ventricular walls that support the papillary muscles, the chordae tendineae, mitral leaflets, annulus, and adjacent left atrial walls. Because defects in any of these components can lead to systolic regurgitation, the list of diseases that can cause mitral regurgitation includes many types of heart disease. Anything that causes left ventricular dilatation may disrupt the alignment of the papillary muscles, impairing their function and dilating the annulus, resulting in mitral regurgitation. Myocardial infarction involving the papillary muscles or the left ventricular walls that support them can impair the function of the mitral apparatus. Mitral chordae can rupture, especially in patients with hypertension or mitral valve pro-lapse. The most common diseases affecting the mitral leaflets are rheumatic heart disease and the myxomatous changes of mitral valve prolapse. In addition, infective endocarditis can destroy the mitral leaflets, and mitral annular calcification can impair the normal systolic contraction of the annulus, leading to mitral regurgitation. Finally, left atrial dilatation from any cause can disrupt annular function and cause mitral regurgitation. Some patients have combinations of these defects, making mitral regurgitation both more likely and more severe.
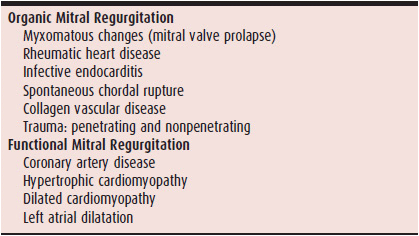
For clinical purposes, mitral regurgitation can be divided into two broad categories: organic and functional. The former refers to diseases that involve the valve leaflets and their immediate supporting apparatus, ie, chordae and annulus. The latter refers to diseases that affect the left ventricle and atrium, leaving the valve apparatus intact (Table 20–1). Most clinical studies involve patients with organic mitral regurgitation, so, unless otherwise specified, the following discussion focuses on organic mitral regurgitation.
Table 20–1. Etiologic Classification of Mitral Regurgitation
Mitral valve prolapse is unique among the many causes of chronic organic mitral regurgitation in many ways. An increase in the middle connective tissue layer of the mitral valve causes an increase in leaflet size and elongated chordae. The resultant systolic prolapse of the valve into the left atrium may or may not be accompanied by regurgitation. In some patients, regurgitation depends on left ventricular volume. Large volumes tend to reduce prolapse and hence regurgitation; small volumes have the opposite effect.
Consequently, the presence or absence of regurgitation and its severity and timing in systole (the ventricle becomes progressively smaller during systole) are determined by a complex interplay of left ventricular volume, pressure, and contractile state. Patients with mitral valve prolapse are also unique because the condition can be hereditary connective tissue disease (eg, Marfan syndrome) or acquired (inflammation of the valve). Some patients exhibit abnormalities of connective tissue in other organs (eg, thoracic skeleton) and have demonstrable abnormalities in the autonomic nervous system. Thus, the clinical presentation of mitral valve pro-lapse varies from one that is similar to other forms of mitral regurgitation to a unique presentation that is dominated by extracardiac manifestations.
In chronic mitral regurgitation, the more common of the two general clinical presentations, the mitral regurgitation progressively worsens as the underlying heart disease worsens. In this situation, the heart has time to adapt to the mitral leak. The increased pressure in the left atrium during systole causes left atrial dilatation. If this is not adequate to decompress the left atrial pressure, the pulmonary arterial tone increases to protect the pulmonary capillaries from increased hydrostatic pressure, resulting in pulmonary hypertension. Because the regurgitated blood returns to the left ventricle in diastole, along with the normal atrial stroke volume, the volume load on the left ventricle results in left ventricular dilatation and eccentric hypertrophy.
Initially, the loading conditions in mitral regurgitation enhance left ventricular performance because preload is increased and afterload is normal. Preload is increased by the augmentation of left ventricular diastolic volume, which increases left ventricular systolic function via the Frank-Starling mechanism. Afterload, or the left ventricular wall tension after the aortic valve opens in systole, is not increased, despite increased left ventricular volume, because much of the increased volume is regurgitated into the left atrium in early systole before the aortic valve opens and because the continued regurgitation during systole reduces forward stroke volume and blood pressure. As the severity of mitral regurgitation increases over time, the ability of the dilated left ventricle to augment systolic function reaches its limits, left ventricular systolic function falls, and heart failure ensues.
Acute mitral regurgitation presents differently because there is insufficient time for these compensatory mechanisms to develop. Sudden rupture of the chordae tendineae, for example, may result in severe acute mitral regurgitation, which markedly increases left atrial pressure. Because the left atrium has no time to dilate, the pulmonary capillary pressure rises markedly, and pulmonary edema usually ensues. The left ventricle also does not dilate adequately to handle the tremendous volume load, and forward failure occurs because of an impaired left ventricular stroke volume. Acute mitral regurgitation (caused by the abrupt failure of a component of the mitral apparatus) can precipitate or aggravate symptoms in a patient with chronic mitral regurgitation.
Nesta F, et al. New locus for autosomal dominant mitral valve prolapse on chromosome 13: clinical insights from genetic studies. Circulation. 2005;112(13):2022–30. [PMID: 16172273]
Magne J, et al. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology. 2009;112:244–59. [PMID: 18758181]
 Clinical Findings
Clinical Findings
A. Symptoms & Signs
1. Chronic mitral regurgitation—The medical history of patients with chronic mitral regurgitation may suggest its cause. Look for a possible history of acute rheumatic fever, coronary artery disease, or a cardiomyopathy. The most common symptom in patients with chronic mitral regurgitation is progressive dyspnea, beginning with dyspnea on exertion and progressing to paroxysmal nocturnal dyspnea and finally orthopnea. Patients may also complain of fatigue and other symptoms associated with congestive heart failure, such as edema. Chronic mitral regurgitation can lead to atrial fibrillation, and palpitations or other symptoms related to this rhythm disturbance may present in patients. Some patients with mitral valve prolapse may have atypical chest pain or inappropriate sympathetic nervous system activation (eg, inappropriate tachycardia, orthostatic hypotension).
2. Acute mitral regurgitation—The patient with acute mitral regurgitation is usually markedly symptomatic, with severe orthopnea or frank pulmonary edema. Although sudden pulmonary edema in itself may suggest the diagnosis, other features of the history may point to the cause of mitral apparatus failure, such as a history of acute myocar-dial infarction, uncontrolled hypertension, or symptoms of infective endocarditis.
B. Physical Examination
1. Chronic mitral regurgitation—In chronic mitral regurgitation, the heart rate may be increased because of atrial fibrillation or heart failure. The carotid pulse is usually brief and of low amplitude, and blood pressure examination shows a narrow pulse pressure. These findings reflect the reduced forward stroke volume. In the presence of heart failure, the respiratory rate may be increased and rales, pleural effusion, edema, increased jugular venous pressure, or ascites may be present. Left ventricular enlargement may result in an enlarged apical impulse. The first and second heart sounds are usually normal, but the pulmonic component of the second heart sound may be increased if pulmonary hypertension is evident. A third heart sound is common because of the left ventricular volume overload, but it does not necessarily indicate heart failure. A fourth heart sound is unusual unless associated coronary artery disease or hypertension is present. The characteristic murmur of chronic mitral regurgitation is usually holosystolic and heard best at the apex, with radiation to the axilla. Occasionally, in patients with posterior leaflet defects, the direction of the regurgitant jet may be anterior, and the murmur is heard in the aortic area. With anterior leaflet defects, the direction of the mitral jet may be posterior and transmitted to the back, where it can be heard up and down the spine. Some reports even note hearing this murmur with the stethoscope on the top of the head. In patients with mitral valve prolapse, the murmur can be crescendo and late systolic. This type of murmur, in fact, almost always represents mitral regurgitation. Often, the late systolic crescendo murmur of mitral valve prolapse is preceded by a midsystolic click from the sudden tensing of the prolapsing leaflets when the end of chordal tethering is reached. Some patients may manifest only a midsystolic click. Occasionally, mitral murmurs will be honking or musical in quality, presumably from a prolapsing leaflet that vibrates in the regurgitant stream. Some patients occasionally have other murmur configurations, whereas others with echocardiographically documented mitral regurgitation have no audible murmur, especially if the regurgitation is mild.
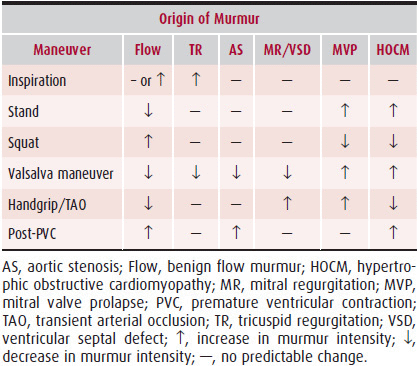
Because murmur configuration and radiation vary in mitral regurgitation, dynamic auscultation is of a great deal of value at the bedside for differentiating this murmur from other heart murmurs. Handgrip exercise is the favored bedside maneuver because it frequently increases the intensity of a mitral regurgitation murmur. In patients with poor grip strength, transient arterial occlusion with two blood pressure cuffs, one on each arm, is useful for producing the same effect (Table 20–2, and Chapter 5). The murmur of mitral valve prolapse behaves like that of mitral regurgitation from any cause, but it has a few unique features. Any maneuver that increases left ventricular volume will (as noted earlier) decrease the amount of mitral valve prolapse and decrease the amount of mitral regurgitation, thereby lessening the intensity of the murmur. Thus, rapid squatting will diminish the murmur of mitral valve prolapse and move the timing of the click-murmur complex later in systole (Figure 20–1). Conversely, maneuvers that decrease left ventricular volume increase the intensity and duration of the murmur of mitral valve prolapse. Thus, standing rapidly from a squatting position will make the murmur louder and move the click-murmur complex toward early systole. Extreme left ventricular volume increases can eliminate the click-murmur and mitral regurgitation, and extreme decrease can result in a pansystolic murmur without a click. Consequently, the auscultatory findings in mitral valve prolapse vary greatly, which can make accurate clinical diagnosis difficult.
Table 20–2. Differentiation of Systolic Murmurs Based on Changes in Their Intensity from Physiologic Maneuvers
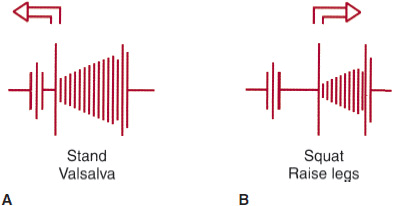
![]() Figure 20–1. Change in the position of the click (C)-murmur (M) complex during systole and the intensity of the murmur as a result of bedside maneuvers. A: Maneuvers that lengthen the murmur and increase intensity. B: Maneuvers that shorten the murmur and reduce its intensity. Note that the position of the midsystolic click also changes with these maneuvers.
Figure 20–1. Change in the position of the click (C)-murmur (M) complex during systole and the intensity of the murmur as a result of bedside maneuvers. A: Maneuvers that lengthen the murmur and increase intensity. B: Maneuvers that shorten the murmur and reduce its intensity. Note that the position of the midsystolic click also changes with these maneuvers.
2. Acute mitral regurgitation—In acute mitral regurgitation, the physical findings are different. The marked increase in left atrial pressure, caused by regurgitation into a non-compliant left atrium, may raise left atrial pressure in late systole to the point that there is no longer any gradient for regurgitant flow. The murmur thus becomes an early systolic murmur rather than the holosystolic murmur characteristic of patients with chronic mitral regurgitation. In fact, when the acute mitral regurgitation is very severe, the murmur may not be audible. In mild-to-moderate acute mitral regurgitation, the murmur responds like the murmur of chronic mitral regurgitation with dynamic auscultation. More severe acute regurgitation is associated with high catecholamine tone and a lack of responsiveness to bedside maneuvers. Other characteristic features of acute mitral regurgitation include a fourth heart sound caused by vigorous atrial contraction following exaggerated expansion during ventricular systole (atrial diastole). In the presence of pulmonary hypertension, the pulmonary second sound increases, and murmurs of pulmonic and tricuspid regurgitation may be present. As mentioned earlier, acute mitral regurgitation invariably results in pulmonary edema. Consequently, the patient will have an increased respiratory rate, diffuse rales, evidence of plural effusion, increased heart rate, a narrow pulse pressure with low systolic blood pressure, and signs of acute right-heart failure, such as increased jugular venous pressure.
3. Mixed valvular disease—Patients with rheumatic valvular disease often have mixed mitral disease, which is defined as at least 2+ (on a scale of 1–4; see following section on Echocardiography) mitral regurgitation, with a mean mitral valve diastolic gradient of more than 10 mm Hg. The clinical course of mixed mitral valve disease is similar to that of mitral regurgitation, and such patients should be treated similarly. Aortic regurgitation frequently occurs with mitral regurgitation, either because of left ventricular dilatation or because the same disease process affects the aortic valve (eg, Marfan syndrome). This places an additional volume load on the left ventricle and usually accelerates the patient’s clinical deterioration.
When aortic stenosis and mitral regurgitation occur together, it is sometimes difficult to ascertain whether the same disease process (eg, rheumatic disease) involved both valves or whether the pressure load of significant aortic stenosis altered left ventricular geometry, performance, or both, resulting in functional mitral regurgitation. Diagnostic imaging studies usually resolve this issue and help direct therapy.
C. Diagnostic Studies
1. Electrocardiography—Patients with chronic mitral regurgitation may have evidence of left ventricular hypertrophy, left atrial abnormality, and sometimes, right ventricular enlargement. Patients with coronary artery disease might have evidence of myocardial infarction or ischemia. Electrocardiographic (ECG) exercise testing is usually done only to confirm the patient’s physical limitations, because ECG changes in the face of a left ventricular volume load are not likely to be accurate for the diagnosis of coronary artery disease. Ambulatory ECG monitoring is occasionally done in patients with palpitations to document atrial fibrillation or other intermittent rhythm disorders.
2. Chest radiography—In cases of chronic mitral regurgitation, an enlarged left ventricle and left atrium would be expected. In severe regurgitation, right-heart enlargement and pulmonary hypertension may be evident. Patients in heart failure will show pulmonary congestion and pleural effusions. In acute mitral regurgitation, there are often signs of pulmonary congestion without enlargement of the heart.
3. Echocardiography—The color-flow Doppler identification of a systolic regurgitant jet across the mitral valve into the left atrium is diagnostic of mitral regurgitation. There are several ways of estimating the severity of mitral regurgitation by analyzing the characteristics of the regurgitant jet on color-flow Doppler. The first method is the depth of penetration of the jet into the left atrium. A penetration of 1 cm or less is considered mild; 2–3 cm, moderate; and 4 cm or more, severe. If the jet is very narrow, the actual volume of regurgitant flow may not be as great as occurs with a more voluminous flow disturbance that penetrates to the same depth. Some investigators have therefore suggested also taking the area of the jet into consideration. One problem with this assessment system, however, is that if the jet impinges on a wall of the left atrium, it appears to penetrate less and be of a smaller area than if it is free in the atrial cavity. There is thus a tendency to underestimate the severity of mitral regurgitation when the jet hits the atrial wall. In addition, a regurgitant jet of any size occurring in a large left atrium will not appear as impressive as the same size jet in a small left atrium. The size of the leak in the mitral valve can be determined by evaluating the jet in a cross-sectional plane at the level of the mitral valve. This method gives an estimate of the size of the hole through which the jet is originating. These qualitative criteria for mitral regurgitation severity can be highly subjective.
For these reasons, there has been interest in more quantitative methods for estimating the severity of mitral regurgitation. The proximal isovelocity surface area observed where flow acceleration and convergence occur on the left ventricular side of the mitral leaflets allows the estimates of regurgitant volume and effective regurgitant orifice area. Fluid dynamics theory states that flow through an isovelocity surface is equal to the velocity times the surface area, which yields instantaneous regurgitant flow. This technique uses the color-flow Doppler color change interfaces observed with accelerating velocity through the orifice to estimate iso-velocity surface area. Pulsed Doppler echocardiography can be used to quantitate regurgitant volume. The principle used is that of flow continuity. Systolic flow out the left ventricular outflow tract represents the forward stroke volume. This can be determined by multiplying the outflow tract area (measured on the two-dimensional echocardiographic image) times the outflow tract systolic velocity–time integral. Flow across the mitral valve in diastole represents the total stroke volume (forward plus regurgitant flow) and can be determined by multiplying mitral annulus area times the diastolic velocity–time integral. Regurgitant volume is the difference between total and forward stroke volume. Typically, the regurgitant volume is reported as the regurgitant fraction, which is the regurgitant stroke volume divided by the total stroke volume. Unfortunately, the calculation of regurgitant stroke volume has many possible sources of error, the largest of which is measuring the flow areas—mitral annular and outflow tract. Thus, individuals without valvular regurgitation can have regurgitant fractions of up to 20%. Also, the calculation of regurgitant flow is time-consuming and requires considerable skill. Effective regurgitant orifice area can be estimated as the ratio of regurgitant volume to the regurgitant jet velocity–time integral by pulsed Doppler.
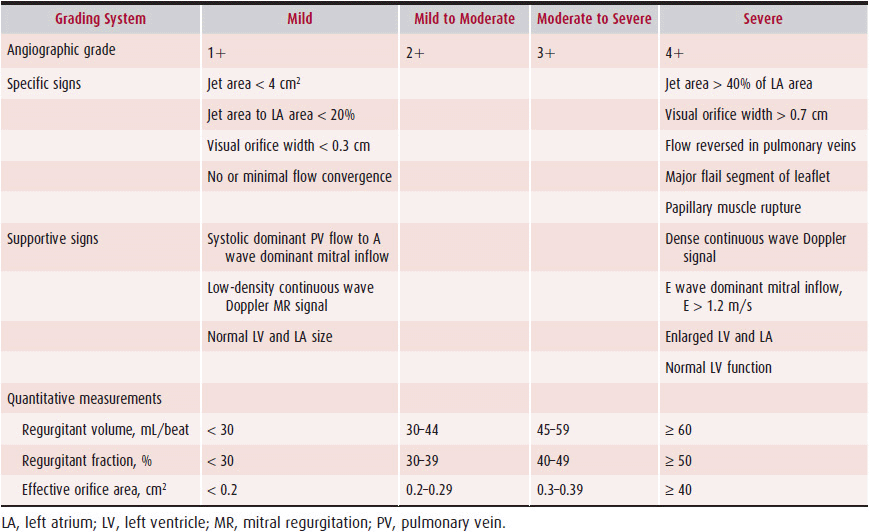
Assessment of pulmonary venous flow velocity by pulsed Doppler echocardiography can also be of value in estimating the severity of mitral regurgitation. Normal pulmonary venous flow velocity is biphasic, with a predominant systolic forward velocity and a lesser diastolic forward velocity in older adults. Systolic forward velocity is reduced in patients with mitral regurgitation, and often the diastolic velocity predominates. In severe mitral regurgitation, the systolic flow in the pulmonary vein signal may reverse. Systolic flow reversal is highly specific for severe regurgitation, but sensitivity is low. Unfortunately, pulmonary venous flow velocity patterns vary considerably in patients with mitral regurgitation, and the predictive value for regurgitation severity is low. In many laboratories, all these factors are integrated to produce a composite estimate of the severity of regurgitation, usually on a scale of 1 to 4, with 4 being severe mitral regurgitation. Table 20–3 exhibits the criteria for grading the severity of mitral regurgitation.
Table 20–3. Echocardiographic Grading of Mitral Regurgitation Severity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


