Minimally Invasive Treatment of Peripheral Vascular Disease: Introduction
Endovascular techniques for treating patients with arterial and venous disorders have revolutionized modern medicine by significantly reducing procedure-related morbidity and hospital costs and delivering outcomes better than or, at least in part, equal to those of open surgical procedures. In the past few years, endovascular therapy has expanded from simple dilatation of atherosclerotic lesions to encompass treatment of aneurysmal arterial disease, acute ischemia, arteriovenous malformations, and venous disorders. Such significant advances in endovascular treatment have given physicians and their patients minimally invasive alternatives to major surgical procedures that carry significant morbidity and mortality. This chapter reviews current advances and explores the benefits and limitations in minimally invasive therapies for the treatment of patients with occlusive and aneurysmal disease of the aorta and peripheral vessels.
Endovascular treatment of peripheral vascular disease (PVD) with balloon catheters was first reported by Fogarty and coworkers1 in 1963. The next year, Dotter and Judkins2 introduced the concept of percutaneous revascularization using coaxial-dilating catheters, followed by Grüntzig and Kumpe’s3 pioneering work that led to the evolution of percutaneous transluminal angioplasty (PTA).
Since then, dramatic advances in balloon and guidewire technology have made it possible to cross difficult lesions and chronic occlusions. Better-designed stents have revolutionized endovascular interventions, providing an attractive and reliable alternative to vascular surgery to the point that endovascular stents are now the standard of care in peripheral vascular interventions. Marked improvement in immediate- and long-term results with stent grafts now permit minimally invasive treatment of aneurysmal disease of the aorta as well as other major vascular territories. Improvements in pharmacologic agents and in catheter-based thrombectomy devices have made endovascular interventions the first-line therapy in patients who have acute limb ischemia (ALI) caused by thromboembolic disease.
Occlusive Disease of the Aortic Arch Vessels
Upper extremity ischemic disease is uncommon, and the majority of cases are secondary to vasospastic disorders, such as Raynaud syndrome and small vessel occlusive disease. In the vast majority of patients who present with chronic symptoms of proximal upper extremity ischemia, atherosclerosis is the underlying cause. Most of these patients are treated medically, with few being candidates for surgical or endovascular therapy.
Although occlusive disease of the subclavian artery is most often asymptomatic because of the rich supply of brachiocephalic collaterals, when it is symptomatic, patients may present with subclavian steal syndrome (vertebrobasilar symptoms that worsen with exercise or work of the ipsilateral upper extremity); ipsilateral upper extremity claudication; or in patients who have an internal mammary artery bypass graft, coronary-subclavian steal syndrome, in which flow to the graft (left internal mammary artery) becomes compromised, leading to angina or myocardial infarction (MI).
Surgical treatment of patients with subclavian artery stenosis (SAS) is effective, but it carries a mortality rate of approximately 2% and a stroke rate of approximately 3%,4 among other complications. The first subclavian artery angioplasty was reported by Bachman and Kim in 1980,5 and although initial reports of using PTA alone showed mixed results with high restenosis rates, recent reports on subclavian artery stenting indicate high procedural success (92%-100%) with good long-term patency (90%-100%).6-9 Although long-term patency data are scant, the endovascular approach is promising and offers many advantages over surgery, including shorter length of stay and lower mortality and morbidity rates.
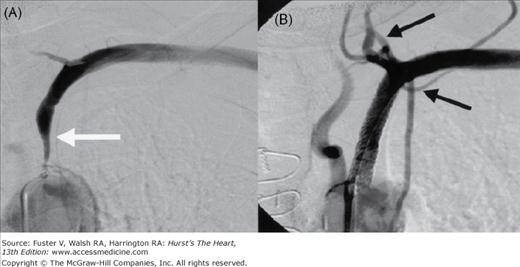
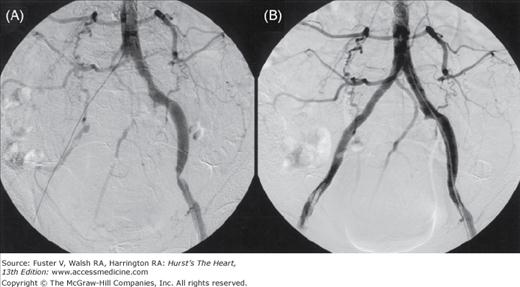
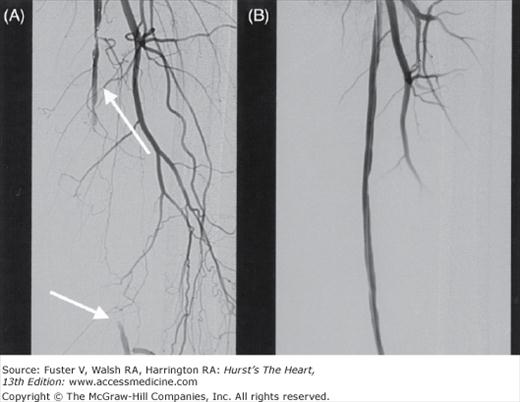
The largest source of data is the report of a multicenter registry9 that evaluated the results of subclavian artery stenting in 258 patients whose primary indications for intervention were arm claudication (43%), subclavian steal syndrome (25%), and compromised flow from the internal mammary graft to the coronary artery (24%), with 86% of the lesions involving the left subclavian artery. Overall, the rate of procedural success was 98.5% with a major complication rate of 1%. At a mean follow-up of 19 ± 15 months, the primary patency rate (PPR) was 89% and the secondary patency rate (SPR) was 98.5%. More recent studies10,11 report similar success. These results suggest that subclavian artery stenting should be considered the primary treatment in patients who need revascularization for SAS (Fig. 111–1).
Figure 111–1.
A 72-year-old woman with dizziness and left arm claudication was found by Doppler ultrasonography to have reversed flow in the vertebral artery (VA). A. Severe left subclavian artery stenosis (white arrow) with no antegrade flow in the VA. B. After stent placement, there is antegrade flow in the VA and left internal mammary artery (black arrows).
Stenosis, particularly at the origin of the vertebral artery, is common, and patients who have occlusive disease of the vertebrobasilar artery (VBA) are at risk for posterior circulation ischemia12 and have an eightfold higher rate of stroke than the normal population.13 The management of VBA stenosis, both at the origin of the VBA and more distally and intracranially, is poorly defined, and surgery for vertebrobasilar ischemia is a relatively rare procedure in most centers where medical treatment has traditionally assumed the major therapeutic role. Surgery is technically challenging, although surgical options such as ostial vertebral endarterectomy, vein patch angioplasty, or reimplantation of the vertebral artery in the subclavian or carotid artery are described. In a series of 290 surgically treated patients, Berguer14 reported no deaths; however, there was a relatively high rate of postoperative complications, including Horner syndrome (15%), recurrent laryngeal nerve paralysis (2%), lymphocele (4%), and immediate thrombosis (1%).
With increasing realization of the poor prognosis of vertebrobasilar disease and advances in endovascular techniques, endovascular intervention is now increasingly performed in this vascular territory. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) is the only completed randomized trial that compared VBA stenting with best medical therapy.15,16 In this study, 16 patients with vertebrobasilar symptoms were randomized to either endovascular therapy or best medical therapy. Technical success in the endovascular group was 100% (five patients had PTA, and three had stents), but transient ischemic attacks (TIAs) occurred in the posterior circulation in two patients within 30 days of the procedure. At 30 days, there were no strokes or deaths in either group. Followed to a mean of 4.5 years in the endovascular group and 4.9 years in the medical group, patients remained free of strokes in the vertebrobasilar territory in both groups. However, limited conclusions can be drawn from this small sample size.
When PTA alone has been used to treat patients who have VBA stenosis,17 vessel recoil and restenosis have resulted in unfavorable long-term results. Recent reports have indicated that stent-supported angioplasty can be performed safely and effectively with low rates of recurrence.18-22 Jenkins and colleagues18 reported a procedural success rate of 100% after vertebral artery stenting in 32 patients (38 arteries) and no deaths at a mean follow-up of 10.6 months. Malek and coworkers21 also reported high rates for procedural success (95.2%) and no adverse events during follow-up.
These reports suggest that stent-supported percutaneous revascularization can be performed in patients who have extracranial VBA stenosis with excellent immediate and long-term results and with very low rates of morbidity and mortality (Fig. 111–2).
American Heart Association/American Stroke Association Council on Stroke Guidelines: Prevention of Stroke in Patients with Ischemic Stroke or Transient Ischemic Attack22
Extracranial vertebrobasilar disease
Class IIB
1. Endovascular treatment of patients with symptomatic extracranial vertebral stenosis may be considered when patients are having symptoms despite medical therapies (antithrombotics, statins, and other treatments for risk factors) (Evidence Level C).
Occlusive Disease of the Aorta and Aortoiliac Bifurcation
Patients who have atherosclerotic occlusive disease of the aorta (Fig. 111–3) and the distal aortic bifurcation and proximal iliac arteries (aortoiliac occlusive disease [AIOD]) can present with lifestyle-limiting claudication, limb-threatening ischemia, or impotence (eg, buttock claudication in patients with erectile dysfunction and absent femoral pulses, a condition termed Leriche’s syndrome). Traditionally, aortoiliac bifurcation disease has been treated with bypass grafting (eg, endarterectomy, aortobifemoral bypass or occasionally axillobifemoral bypass, iliobifemoral bypass, and femorofemoral bypass), which yields an excellent long-term outcome but has been associated with a perioperative mortality rate up to 2% to 4% and a rate of major early complications (including sexual dysfunction, ureteral damage, intestinal ischemia, and spinal cord injury) of 5% to 13%.23
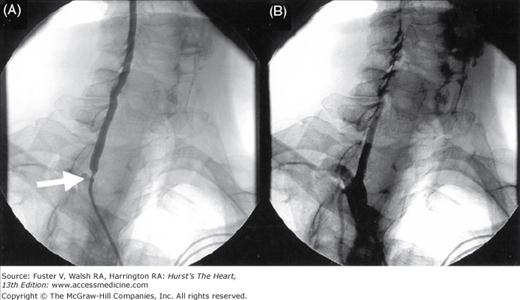
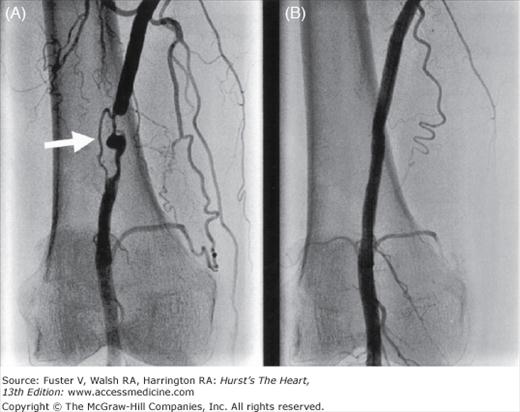
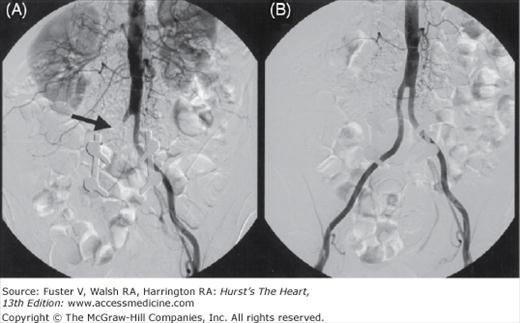
Figure 111–3.
Angiograms in a 65-year-old woman with a history of severe claudication and peripheral vascular disease after surgical bypass. A. Selective abdominal aorta angiography showed severe stenosis below the renal artery at site of surgical clamping (arrow) B. After balloon and stenting of abdominal aorta with resolution of gradient.
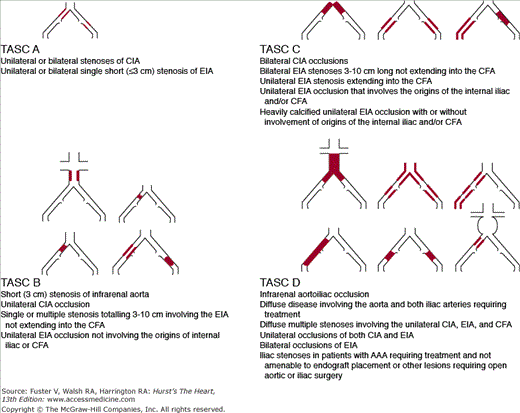
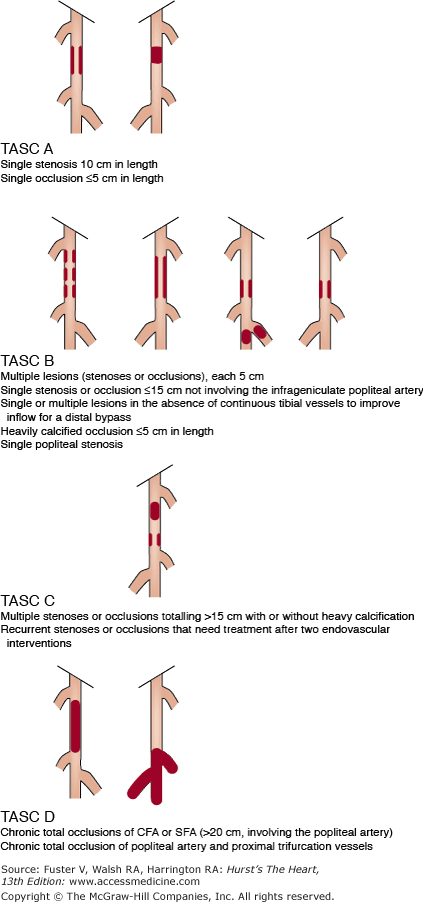
The Transatlantic Inter-Society Consensus (TASC II) classifies aortoiliac lesions into TASC A through D (Fig. 111–4). Advances in endovascular techniques have allowed endovascular treatment to rapidly replace open surgery in mild to moderate lesions, defined as TASC class A and B lesions,24 although controversy surrounds the best course of action in cases of severely calcified, long-segment, or extensive occlusion of the iliac arteries or the entire aortic bifurcation (TASC class C and D lesions). In general, however, endovascular therapy has become an established, safe (mortality, 0%-2.3%), and effective method in the treatment of patients with AIOD. In fact, AIOD represents one of the best indications for PTA with or without stenting. An exhaustive meta-analysis of multiple published studies by Kandarpa et al25 showed that iliac balloon angioplasty had a PPR of up to 86% at 1 year and 74% at 4 years. When stenting was used additively, the PPR improved to 91% at 1 year and 78% at 4 years.
Although PTA of the ostium of the common iliac artery sometimes causes plaque to shift across the aortic bifurcation, producing stenosis in the contralateral iliac artery,26 the kissing balloon technique, which involves positioning two balloons across the origins of both iliac arteries and inflating these balloons simultaneously, can largely avoid these complications. This technique has good rates of procedural and clinical success, including lower mortality rates; however, the incidence of significant residual stenosis, dissection, thrombosis, or distal embolization can be up to 9%.26,27 These complications can be minimized by using a kissing stent technique (ie, simultaneous deployment of stents at the aortoiliac bifurcation). Our results and those of others have been excellent, both immediately postprocedure and long term (Fig. 111–5).28-32 Our rate of procedural success has been 100% with rate for primary patency of 92% and SPR of 100% during follow-up of longer than 18 months. Although acute complications (distal embolization) occurred in 4%, our patients had no vascular complications, MIs, or perioperative deaths. Usual indicators for failure of endovascular therapy33-36 in the aortoiliac segment are shown in Table 111–1.
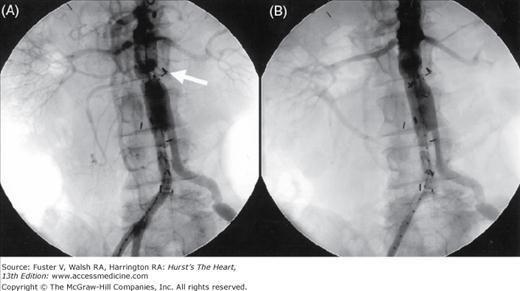
Figure 111–5.
Angiograms in a 71-year-old man with severe lifestyle-limiting claudication. A. Angiography showed occluded right common iliac artery and moderate lesion in the left common iliac artery and severe lesion in the left external iliac artery. B. Self-expanding SMART Control stents (Cordis Corp., Bridgewater, NJ) deployed with a kissing technique followed by postdilatation using kissing balloons.
| 1. Small iliac artery diameter |
| 2. Concomitant external iliac artery disease |
| 3. Need for thrombolysis |
| 4. Complication during the procedure |
| 5. Focal lesions >2-3 cm |
| 6. Occlusive lesions |
| 7. Critical limb ischemia |
| 8. Calcified lesions |
| 9. Poor distal runoff |
| 10. History of diabetes mellitus |
In summary, revascularization of AIOD has shifted from a predominantly surgical to an endovascular-based therapeutic approach, which has become the clinical standard of care in aortoiliac disease.37 Currently, in the treatment of patients with AIOD, several specialties are involved with the care of these patients, including vascular surgeons, interventional radiologists, and interventional cardiologists, and the long-term outcomes of patients with this distribution of disease treated with endovascular approach are excellent.
Occlusive Disease of the Iliac Arteries
When long-term outcomes from recent trials in patients with iliac stents are combined, an acute procedural success rate of more than 90% is seen, with 3 ± 1-year PPRs of 74% to 87% and SPRs of 84% to 95%, a serious complication rate of 1.4%, and a 30-day mortality risk of 0.5% (compared with 4% for aortofemoral bypass). Thus, patency rates of primary stenting compare favorably with surgical patency rates while carrying a lower risk of mortality and morbidity.28,38-41 Aortofemoral bypass, the traditional surgical treatment for patients with iliac occlusive disease, carries a mortality rate of up to 5%, an early graft failure rate of 5.7%, and a patency rate after 2 years of 92.8%.42
The iliac arteries are best suited for the endovascular approach because of their ease of percutaneous access, high-flow state, and relatively large diameter, which improves expected immediate technical results and yields excellent postprocedural rates of long-term patency. The indicators for good results from PTA in this segment are shown in Table 111–1. In patients who fit these criteria, the initial technical success rate can be more than 90% with a 5-year PPR of approximately 80%.43 In those who have longer, calcified lesions or occluded arteries, the success rate is lower, in which case intravascular stents can be used with excellent results. In fact, current guidelines recommend primary stent placement in the iliac arteries.44
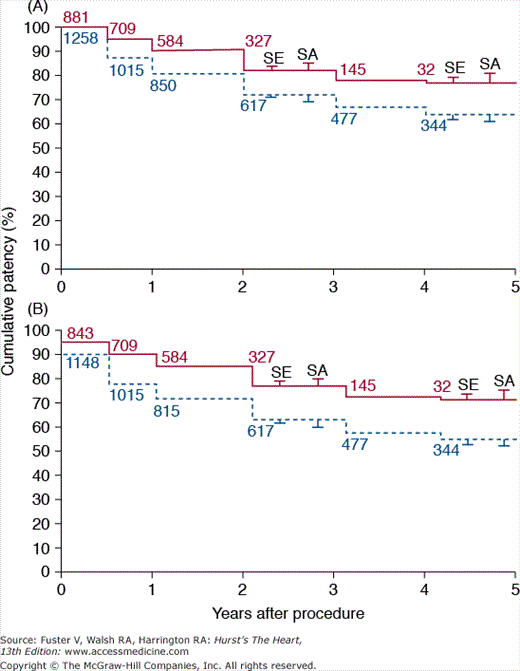
Several studies have investigated the role of endovascular stents in treating iliac artery occlusive disease.28,45-48 In a meta-analysis (14 studies; >2000 patients) of PTA versus PTA plus stenting, Bosch and Hunink46 concluded that PTA with stent placement lowered the risk of long-term failure by 39% compared with PTA alone (Table 111–2 and Fig. 111–6). Currently, endovascular stents have become an accepted therapy for patients with iliac occlusive disease. Vorwerk and colleagues,28 using self-expanding WALLSTENT RX Biliary Endoprosthesis (Boston Scientific, Natick, MA) to treat 109 patients who had iliac artery stenoses after failed PTA, reported a PPR of 82% and SPR of 91% at 4 years. In a multicenter study47 (486 patients) using Palmaz-Schatz Crown balloon-expandable stents (Cordis Corp., Bridgewater, NJ), the rate of technical success was 99%, and the rate of clinical patency at 2 years was 84%.
| PTA | PTA + Stent | |||
|---|---|---|---|---|
| Stenosis | Occlusion | Stenosis | Occlusion | |
| Immediate technical success (%) | 96 | 80 | 100 | 80 |
| Primary patency (%) | 65 | 54 | 77 | 61 |
| Secondary patency (%) | 80 | 80 | ||
| Major complications (%) | 4.3 | 5.2 | ||
Figure 111–6.
Cumulative primary patency curves after percutaneous transluminal angioplasty PTA (dashed blue line) and stent placement (solid red line), unadjusted for covariates and excluding technical failures (A) or including technical failures (B). Numbers indicate the number of patients entered in each interval during follow-up. In addition, the standard error (SE) and the range found in the sensitivity analysis (SA) are given. Reproduced with permission from Bosch and Hunink.46
Scheinert and coworkers48 evaluated the role of primary stenting after excimer laser-assisted recanalization in 212 patients who had chronically occluded iliac arteries. These authors reported a rate of technical success of 90% and a complication rate of 1.4% (arterial rupture or embolism) with rates of primary patency of 91% at 1 year, 84% at 2 years, and 76% at 4 years. Surgical and percutaneous treatment options for TASC class B and C lesions were reported in a nonrandomized observational study by Timaran et al,49 and no difference in limb salvage or survival at 5 years was found, although limited or compromised runoff seemed to predict higher failure rates. In two randomized, controlled trials of surgery versus angioplasty for iliac occlusive disease and limb-threatening ischemia, no differences in 1- or 3-year cumulative rates for death, amputation, or revascularization failure were reported.50,51
With regard to the choice of stent selected for therapy, the Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) trial52 found no difference in patency of self-expanding nitinol stent (an alloy of nickel and titanium) versus balloon-expandable stainless steel stent. Thus, current evidence suggests the type of stent used in iliac artery intervention does not necessarily impact immediate or long-term success and that the choice of stent is usually individualized as determined by the extent, nature of the lesion, ideal location of intervention, and operator’s experience level with a particular stent system.
Iliac artery PTA and stenting is also used as an adjunct to peripheral vascular surgery. For example, it may help to facilitate the patency of a downstream surgical conduit during surgical revascularization for femoropopliteal disease. In patients who are at high risk for vascular surgery because of concomitant coronary artery disease, it may provide a less invasive femoral bypass compared with a high-risk surgical procedure such as aortobifemoral bypass. Thus, iliac artery PTA and stenting is well supported by current data as the initial choice for treating patients who have iliac occlusive disease for its reduced invasiveness, excellent rates of technical success, and good rates of long-term patency.
Occlusive Disease of the Femoropopliteal Arteries
Atherosclerotic occlusive disease is two to five times more frequent in femoropopliteal arteries than in iliac arteries and more common in this arterial segment than any other vascular bed in the body (Fig. 111–7). Typically, the atherosclerotic lesions tend to be multiple, long, ulcerated, and frequently associated with dense calcification. Patients vary in their clinical presentation from intermittent claudication to rest pain and critical leg ischemia. In addition, acute-onset ischemia is much more frequent in patients with femoropopliteal artery disease than those with iliac artery disease. Although choices for managing iliac artery disease are clear cut and proven, choices for managing disease in the femoropopliteal arteries are not as durable, and both surgical and endovascular forms of treatment have been plagued by treatment failure. Also, the optimal choice of therapy (endovascular vs surgical) continues to be a question of debate because long-term patency remains elusive. Although opinions vary over the choice of therapy in patients with claudication, some form of intervention is imperative when pulsatile flow must be restored to prevent limb loss in patients who have rest pain or leg ischemia.
Randomized trials comparing PTA and bypass surgery (BPS) in the infrainguinal segment are sparse, probably because BPS has been commonly performed in extensive disease with long lesions and critical limb ischemia (CLI), but PTA has been more commonly performed in limited disease and short obstructions (following the original TASC I recommendations). The BASIL (Bypass versus Angioplasty in Severe Ischemia of the Leg) trial prospectively assigned patients with severe limb ischemia caused by infrainguinal disease to undergo either angioplasty or BPS. At the end of the 5.5-year study, there was no difference between the two groups in survival to primary endpoint (amputation-free survival; 68% for the surgical group and 71% for the PTA group) or at 3 years (57% for the surgical group and 52% for the PTA group). However, in the angioplasty group, rates for morbidity and length of hospital stay were significantly lower, and the total cost was significantly less.53
Similarly, Wolf and colleagues,54 in a multicenter, prospective, randomized trial comparing PTA with BPS for treatment of iliac, femoral, or popliteal artery obstruction in 263 men, showed no significant difference in outcomes over a median follow-up of 4 years (survival, patency, and limb salvage). Hunink and colleagues55 compiled a useful analysis of the literature that compares the outcomes of PTA with those of BPS and helps identify patient subgroups that will benefit most from each treatment modality. Despite study limitations, they concluded that successful PTA depends on lesion type (ie, stenosis vs occlusion) and on the patient’s having good distal runoff vessels. With good distal runoff vessels, the patency rate in stenotic vessels at 5 years was 56% to 63%, decreasing to 35% to 48% if the treated vessel was occluded or if runoff was poor and to 19% to 22% if there was both an occluded vessel and poor runoff.
In the case of the common femoral artery (CFA), percutaneous revascularization is challenging because of the anatomical location of the vessel (ie, over the hip joint, where stent placement may be suboptimal and stent fracture becomes a concern). Therefore, occlusive disease of the CFA is best treated with an open-surgical approach (iliofemoral bypass or endarterectomy with patch angioplasty) and is associated with a more than 90% success rate (1-year patency rate, 80%) but with a heavy burden of postoperative morbidity (infection, hematomas, seromas) in more than 15% of patients.56,57 Silva and colleagues58 recently reported their experience with provisional stent placement in CFA disease in a small series of patients. They achieved a procedural success rate of 95% and event-free survival, including target vessel revascularization and amputation of 90% to 95% at 1-year follow-up. However, acute limb-threatening ischemia, with acute vessel closure leading to immediate limb loss, remains a concern. Similarly, the profunda femoris artery has historically been revascularized by surgical treatment, although “percutaneous profundaplasty,” described by Silva and colleagues,59 was shown to achieve an immediate hemodynamic success rate of 97% with an in-hospital limb salvage rate of 94% and 88% at 3- and 5-year follow-up, respectively, using provisional stenting with or without thrombolytic therapy.
The case of femoropopliteal arterial segment has been a focus of great interest recently for endovascular interventions, and current procedural success for treatment of stenotic femoropopliteal occlusive disease and chronic total occlusion (CTO) is high. The advent of hydrophilic guidewires and catheters has particularly enhanced our ability to traverse occlusions in these segments, resulting in success in 80% to 90% of cases.60-62 The use of devices such as the Pioneer, Outback, and Frontrunner (reentry catheters), designed to facilitate controlled passage into the true arterial lumen after subintimal guidewire passage during recanalization procedures, has been associated with an 80% to 100% technical success rate in crossing the desired occluded segment.63,64 Consequently, even though rates of long-term patency are far lower than those in the iliac arteries, PTA is increasingly used to treat disease in femoropopliteal arteries.
Traditionally, percutaneous treatment of superficial femoral artery (SFA) by balloon angioplasty or stent placement has been perceived to have suboptimal long-term results based on many small clinical trials24,60-62 and earlier meta-analyses.65,66 For example, a meta-analysis by Kandarpa and colleagues65 demonstrated a 1-year patency rate for PTA of 26% to 79%, declining to 12% to 68% at 5 years. The TASC II report calculated a weighted PPR for stenosis and occlusions in the femoropopliteal system together, which showed suboptimal results (Table 111–3). The reason for these unclear benefits of endovascular therapy in femoropopliteal lesions is probably attributable to the small number of patients studied, differences in patient population, lesion characteristics, and differences in type of stent used, making useful comparison among studies impossible. However, recent studies have been much more encouraging. Table 111–4 shows an overview of the recently completed trials in SFA revascularization.67,68 Preliminary results of ongoing clinical studies on SFA stenting are also encouraging. For example, the RESILIENT (Randomized Study Comparing the Edwards Self-Expanding LifeStent vs Angioplasty alone In LEsions INvolving The SFA or Proximal Popliteal Artery) trial69 reported a 6-month PPR (mean lesion length, 7 cm in the stent group and 6.4 cm in the BA group) of 94% versus 47% 1-year patency of 81% versus 37% for the stent group compared with the balloon angioplasty group (P <.0001 in both cases). Freedom from target lesion revascularization (TLR) at 1 and 2 years was recently reported70 as 72% for primary stenting versus 32% for PTA alone and 68% for stenting versus 24% for PTA alone (P <.0001 in both cases). However, results of the RESILIENT trial may be hard to extrapolate in clinical practice settings where patients with longer stenotic and occlusive lesions (≥10 cm) are encountered routinely and may therefore need longer stents or more than one stent. Indicators that adversely affect the degree of patency in femoropopliteal arteries after endovascular approach are shown in Table 111–5.
| 1-Year % Patency (range) | 3-Year % Patency (range) | 5-Year % Patency (range) | |
|---|---|---|---|
| PTA: stenosis | 77 (78-80) | 61 (55-68) | 55 (52-62) |
| PTA: occlusion | 65 (55-71) | 48 (40-55) | 42 (33-51) |
| PTA + stent: stenosis | 75 (73-79) | 66 (64-70) | |
| PTA + stent: occlusion | 73 (69-75) | 64 (59-67) |
| Trial | Schillinger 2006 (Dynalink)a | Schillinger 2007 (Absolute/Dynalink)b | FAST (Luminexx)c | FACT (Conformexx)d | Ferriera (Zilver)e | |||
|---|---|---|---|---|---|---|---|---|
| Characteristics | PTA | Stent | PTA | Stent | PTA | Stent | Stent | Stent |
| Patients (n) | 53 | 51 | 52 | 46 | 121 | 123 | 110 | 59 |
| Lesion type/length (mm) | 92 ± 64 | 101 ± 75 | 93 | 112 | 51.1 ± 24 | 53.4 ± 29.5 | 59.1 ± 58.7 | |
| Occlusion (%) | 17 | 19 | 16 | 19 | 30 | 45 | 35 ± 5 | NR |
| PPR at 1 y (%) | 67 | 76 | 31.8 | 54 | 61.5 | 69.3 | 76.7 | 90 |
| Target lesion revascularization | NR | NR | NR | NR | 18.3% (1 y) | 14.9% (2 y) | 7.4% (2 y) | NR |
| Negative Indicators | Positive Indicators |
|---|---|
| Occlusion, especially >10 cm | Absence of occlusion |
| Calcified vessels | Absence of calcification |
| Multiple lesion segments | Single lesion |
| Rest ischemia | Pain only during activity |
| Poor distal runoff | Good distal runoff |
Also, in the femoropopliteal arteries, use of balloon-expandable stents is no longer recommended because of high rates of stent deformation from crush injury and because of poor rates of long-term patency. At present, only self-expanding stents and covered stent grafts (Viabahn, W.L. Gore & Associates, Inc. Flagstaff, AZ) (Figs. 111–8 and 111–9) are used for treating occlusive disease in femoropopliteal arteries.
Covered stents or stent grafts (self-expandable metal stents covered with a semipermeable or nonpermeable material), such as the expanded polytetrafluoroethylene-covered nitinol stent graft Viabahn endoprosthesis, are increasingly used to treat long-segment SFA lesions in the hope of reducing restenosis rates and improving long-term patency in occluded femoropopliteal vessels. It is believed that the stent covering prevents neointimal ingrowth through the interstices of the stent along the treated segment. The efficacy of the covered stent grafts have been reported earlier by Dorrucci,71 Kedora et al,72 Kazemi et al,73 and Alimi et al,74 and now more recently by McQuade and colleagues.75 The latter prospectively randomized 100 limbs in 86 patients with SFA occlusive disease (all TASC A, B, C, and D lesions) to treatment with Viabahn stent graft versus femoral-to-above-knee-popliteal artery bypass with synthetic graft material and followed them for 24 months. The stent-graft group demonstrated a PPR of 81%, 72%, and 63% with an SPR of 86%, 83%, and 74% at 6, 12, and 24 months, respectively. The surgical femoral-popliteal group demonstrated a PPR of 84%, 77%, and 64% with an SPR of 89%, 86%, and 76% at 6, 12, and 24 months, respectively (P = .716 and .695 for PPR and SPR, respectively), thus demonstrating that management of SFA occlusive disease with percutaneous stent grafts exhibits similar primary patency at 24-month follow-up compared with conventional femoral–popliteal artery bypass grafting with synthetic conduit. This further emphasizes that in contemporary clinical practice with advanced endovascular techniques and equipment, a suitable alternative may exist in the treatment of the superficial femoral artery segment for revascularization when prosthetic bypass is being considered or when autologous conduit is unavailable.
The Viabahn stent graft has also been used in the treatment of in-stent restenosis (ISR). Djelmami-Hani and colleagues76 used stent grafts for ISR with measurable success. They followed 26 patients who underwent 32 endovascular repairs of ISR lesions of the SFA using Viabahn stent grafts. All patients were followed closely with duplex scans at 1, 3, 6, and 12 months postprocedure, and although technical success rate was 100% with no in-hospital mortality or morbidity at a mean follow-up of 20 ± 10.6 months, the PPR was 59.4%, and the SPR was 65.6%. There were six (18.7%) cases of total stent-graft occlusion at mean follow-up. If these results are replicated in large-scale randomized trials, minimally invasive therapy may become the treatment of choice for patients with femoropopliteal disease. The Gore VIABAHN Endoprosthesis veRsus bAre Nitinol stent (VIBRANT) study is one such ongoing randomized, prospective, multicenter clinical trial comparing the Viabahn device with bare nitinol stents at 15 study sites over 3 years; preliminary results are expected soon.
Other devices such as the SilverHawk extraction atherectomy device, excimer laser atherectomy device, cutting balloon atherotomy device, and PolarCath cryotherapy device are also available for endovascular treatment of infrainguinal disease and use the principles of debulking, cryoplasty, and brachytherapy. Most such devices are used in SFA occlusive disease and, although none of them have been tested in randomized clinical trials, preliminary data on these devices have been somewhat promising,77-89 but their role is not fully defined yet in SFA disease.
Although drug-eluting stents (DES) are broadly accepted and used in endovascular coronary procedures, experience and evidence with DES in PVD is limited.90-92 The SIROCCO (SIROlimus Coated Cordis smart stents for the treatment of Obstructive SFA disease) trial,90,91 the first randomized, controlled trial that evaluated a DES (self-expanding nitinol stents coated with a polymer impregnated with sirolimus) against a bare-metal stent (BMS), failed to show clinical or angiographic endpoint improvement using bare-metal nitinol stent over sirolimus-eluting nitinol stent in the femoropopliteal arteries. Currently, results are awaited on two major DES trials in the treatment of infrainguinal disease: the STRIDES (SFA TReatment wIth Drug-Eluting Stents study) trial and the Zilver PTX study.
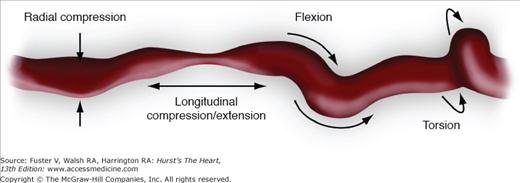
Compared with patients with aortoiliac disease, those with infrainguinal disease are much more difficult to treat and, unlike the success achieved in the aortoiliac segment using PTA with or without stenting, results of endovascular therapy in the infrainguinal vessels have not been as durable. This is partly because of the multiple levels of occlusive disease encountered in the infrainguinal vessels and because of the unique biomechanical forces (Fig. 111–10) that plague this segment of the arterial tree, leading to stent compression and fracture that may result in thrombosis, early restenosis, and pseudoaneurysm formation. This is true for both balloon-expandable stents and self-expanding stents. In the near future, newer stent designs and stent grafts may improve the results of stenting in the femoropopliteal area. The role of stenting and DES in the treatment of patients with SFA occlusive disease will become clearer when the final results are released from the RESILIENT study, the Zilver PTX trial, and the STRIDES trial.
In summary, the current consensus is to (1) recommend endovascular revascularization in symptomatic patients for femoropopliteal TASC A lesions; (2) prefer it for TASC B and C lesions over open surgical repair; and (3) consider it in patients with TASC D lesions who are considered high-risk surgical patients (see Fig. 111–7). In addition, in patients with CLI, based on the BASIL study results, endovascular revascularization is the preferred option over open surgical repair.
Occlusive Disease of the Infrapopliteal Arteries
There are three main differences between PVD involving the infrapopliteal arteries and disease that involves the other peripheral arteries:
When an iliac artery or SFA is occluded or has critical stenosis, the result may be lifestyle-limiting claudication or limb ischemia. But in isolated below-the-knee disease, having only one patent infrapopliteal artery is enough to maintain blood flow and prevent limb ischemia despite disease in other infrapopliteal arteries.
In isolated below-the-knee disease, especially in patients with diabetes and renal failure, ankle-brachial indices (ABIs) are unreliable for assessing symptoms or for determining disease severity because of the noncompressibility of these vessels.
Treatment of infrapopliteal disease is difficult given the diffuse nature of the disease and the presence of inflow disease. With infrapopliteal occlusion, the restenosis rate after angioplasty is reportedly as high as 40% to 60% and is related primarily to diffuse disease; the presence of small-diameter vessels; and, frequently, to long calcified lesions.
The immediate and long-term results of arterial reconstruction for infrapopliteal disease are better in patients who have claudication than in those who have CLI. However, tibial artery BPS has traditionally been reserved for selected patients who have CLI to achieve revascularization distal to infrapopliteal obstructions. Although data have not been encouraging,93-95 Veith and coworkers93 used innovative and creative techniques to improve results, dramatically decreasing rates of procedure-related amputation from 49% to 14%. Their distal bypass procedures, however, resulted in a coincident 30-day mortality rate of 4% and a 90-day graft failure rate of nearly 5%. These results led to acceptance of tibial artery BPS as the standard of care in the 1990s, with success defined as clinical improvement with resolution of rest pain. All the same, use of percutaneous intervention is a more attractive alternative when graft patency deteriorates as a result of distal anastomoses to small diseased vessels, diffuse distal arterial occlusive disease produces poor runoff, or grafts have to cross a joint.
Currently, endovascular intervention for infrapopliteal disease is increasingly being undertaken as newer low-profile balloons and new-generation wires become available and improve the success rate of infrapopliteal angioplasty for treating occlusions and stenotic lesions. The primary goal of infrapopliteal arterial intervention for CLI is to restore at least one unobstructed line of blood flow to the distal foot, and success of endovascular therapy in this area is usually measured by relief of rest pain, improved ulcer healing rate, and avoidance of amputation. Several reports96-109 have now demonstrated the feasibility, safety, and efficacy of tibioperoneal PTA combined with atherectomy and stenting as adjuvant procedures. Primary procedural success rates are high for ideal lesions, and cumulative 2- to 5-year limb salvage and patency rates of 80% to 90% can be achieved with modern endovascular techniques rivaling those of surgical reconstruction.110,111 The technical success rates for infrapopliteal angioplasty range between 78% and 100%,106,108,112-114 and a random-effects meta-analysis demonstrated a technical success rate of 93% and 1- and 2-year limb salvage rates of 79% and 74%, respectively.106,115 In comparison, the limb salvage rates for distal bypass at 1 and 2 years are 79% and 74%, respectively, and up to one-third of surgical patients require repeat intervention to maintain graft patency.106 In our published study of 97 patients who had lifestyle-limiting claudication, CLI or both, the success rate was 95%, including an 86% rate of successful limb salvage.116 These reports demonstrate that patients who have CLI caused by infrapopliteal disease should be seriously considered for endovascular procedures as an alternative to surgery (Fig. 111–11).
Figure 111–11.
Angiograms in a 67-year-old man with a history of nonhealing ulcer in the left foot. A. Angiography showed severe below-the-knee disease with occluded anterior tibialis artery, a severely diseased tibioperoneal trunk (white arrow), and an occluded posterior tibial artery (black arrow). B. After percutaneous balloon angioplasty of the tibioperoneal trunk and posterior tibial artery.
Although long-term data regarding the benefit of stent use in below-knee disease are lacking, the use of stenting in the tibioperoneal trunk in an effort to increase patency rates and limb salvage has been undertaken, and a few reports96,117-119 describe the application of stents in the arteries below the knee. Feiring and colleagues118 reported that below-knee stent-supported PTA for CLI not only improved ABI more than tibial bypass, but it also healed ulcerations, relieved rest pain, and improved ambulation. Siablis and colleagues,96 in a recent small, prospective, randomized trial of angioplasty versus stenting using a carbon-coated stent (carbo-stent), showed better 6-month angiographic patency rates for stenting (84% vs 61% using a 70% diameter threshold on computed tomography [CT] angiography measurements). Even DES has proven effective in this application with sirolimus-eluting stents already having demonstrated marked short-term angiographic and clinical efficacy in the infrapopliteal arteries.96,119-121 Recently, Siablis and colleagues,96 in a nonrandomized comparison of tibioperoneal stenting in 58 patients (28 BMS and 29 SES), demonstrated a marked reduction in restenosis at 6 months from 55% in BMS to 4% in the DES category. This led to the approval of the balloon-expandable sirolimus-eluting stent CYPHER (Cordis Corp., Bridgewater, NJ) in Europe for this indication.
Varying rates of clinical success have also been reported with other new devices, including directional atherectomy catheters and laser-assisted angioplasty. Zeller and coworkers122 reported successful use of the SilverHawk device in below-knee arteries. Their procedural success rate was 98% with significant improvement in ABIs postprocedure. The 6-month cumulative patency rate was 98%. Laird and colleagues123 reported use of laser-assisted angioplasty in 145 patients with 155 critically ischemic limbs. Procedural success was seen in 86% of the patients with limb salvage achieved in 92% of the patients.
In our view, there has been a paradigm shift in the management of tibioperoneal occlusive disease, and endovascular revascularization in this territory is both safe and effective in this high-risk cohort of patients. When anatomically feasible, PTA is now considered a reasonable and appropriate first option for revascularization of all patients, even those with low surgical risk for infrapopliteal bypass.37 In addition, the availability of effective and less invasive options currently permits a lower threshold for intervention in such cases, and more so in that subset of patients who claudicate solely based on the infrapopliteal nature of their disease.
Endovascular Treatment of Claudication: Current American College of Cardiology/American Heart Association Practice Guidelines44
Class I
Endovascular procedures are indicated for individuals with a vocational or lifestyle-limiting disability caused by intermittent claudication when clinical features suggest a reasonable likelihood of symptomatic improvement with endovascular intervention, and (a) there has been an inadequate response to exercise or pharmacological therapy, and/or (b) there is a very favorable risk-benefit ratio (eg, focal aortoiliac occlusive disease).
Endovascular intervention is recommended as the preferred revascularization technique for TASC type A iliac and femoropopliteal arterial lesions.
Stenting is effective as primary therapy for common and external iliac artery stenoses and occlusions.
Class IIa
Stents (and other adjunctive techniques, such as lasers, cutting balloons, atherectomy devices, and thermal devices) can be useful in the femoral, popliteal, and tibial arteries as salvage therapy for a suboptimal or failed result from balloon dilation (eg, persistent translesional gradient, residual diameter stenosis greater than 50 percent, or flow-limiting dissection).
Class IIb
The effectiveness of uncoated/uncovered stents, atherectomy, cutting balloons, thermal devices, and lasers for the treatment of infrapopliteal lesions (except to salvage a suboptimal result from balloon dilation) is not well established.
Class III
Endovascular intervention is not indicated if there is no significant pressure gradient across a stenosis despite flow augmentation with vasodilators.
Primary stent placement is not recommended in the femoral, popliteal, or tibial arteries.
Endovascular intervention is not indicated as prophylactic therapy in an asymptomatic patient with lower-extremity PAD.
Endovascular Treatment for Acute Limb Ischemia
ALI is a true medical emergency and occurs when a sudden decrease in blood flow to a limb threatens its viability. By definition, it is of sudden onset (⩽14 days) and patients present with symptomatic, diminished, or worsening limb perfusion that endangers both limb and life (Table 111–6). The cause of ALI commonly involves a major artery or a bypass graft that is acutely obstructed by either an embolus (often from the heart) or thrombosis in situ. The principal goal of treatment for ALI is to rapidly restore blood flow to the ischemic region to forestall irreversible changes. Immediate surgical revascularization (thromboembolectomy, endarterectomy, bypass, graft revision) is usually indicated for the profoundly ischemic limb (sensory or motor deficits of very short duration), but recent advances in endovascular management have changed practice patterns. Catheter-directed thrombolysis (CDT), a minimally invasive endovascular technique, has been shown to be useful for rapid clot dissolution, unmasking underlying stenoses, and helping to determine the best treatment strategy (either surgery or PTA).124 CDT offers the following advantages37 when compared with surgical revascularization:
Lower mortality
Less complexity of the procedure
Reperfusion achieved at a lower pressure
Lower risk of reperfusion injury
Further definition of the underlying lesions by angiography with appropriate adjunctive management
Reduced risk of endothelial trauma and clot lysis in branch vessels too small for embolectomy balloons.
| Doppler Signals | ||||||
|---|---|---|---|---|---|---|
| Category | Description | Capillary Return | Muscle Weakness | Sensory Loss | Arterial | Venous |
| Viable | Not immediately threatened | Intact | None | None | Audible (AP > 30 mm Hg) | Audible |
| Threatened | Salvageable if promptly treated | Intact, slow | Mild, partial | Mild, incomplete | Inaudible | Audible |
| Irreversible | Major tissue loss; amputation regardless of treatment | Absent (marbling) | Profound, paralysis (rigor) | Profound, anesthetic | Inaudible | Inaudible |
Studies have shown that use of CDT leads to long-term clinical outcomes equal to those of surgical revascularization in treating limb-threatening ischemia.125-127 CDT is based on the principle that activation of fibrin-bound plasminogen to the active enzyme plasmin is the most effective means of lysing thrombi, and direct delivery of a thrombolytic agent produces increased activity at the desired location and permits effective thrombolysis at a reduced dose. Three randomized trials—Rochester, STILE (Surgery versus Thrombolysis for Ischemia of the Lower Extremity), and TOPAS (Thrombolysis Or Peripheral Arterial Surgery) I and II125-127—compared intrathrombotic thrombolysis of urokinase or recombinant tissue-type plasminogen activator (rt-PA) with initial surgical intervention and confirmed the role of CDT as first-line treatment for ALI. The prospective, randomized STILE trial reported no difference in rates of mortality, amputation, or major morbidity in groups treated surgically and those treated with thrombolysis (urokinase or rt-tA).125 The rates of limb salvage were also similar (88.2% in the surgical group vs 89.4% in the thrombolysis group).
The TOPAS I and II studies found no difference in rates for mortality and for amputation between groups treated with urokinase and those treated with surgery, but the magnitude of surgery was reduced in the thrombolysis group.126,127 Nonrandomized trials have demonstrated that thrombolytic therapy followed by PTA may obviate bypass grafting in more than 50% of patients.128 Accordingly, unless patients present with critical ischemia that demands immediate restoration of pulsatile blood flow by surgical embolectomy (patients with loss of motor and sensory function in a viable limb), CDT should be the initial therapy of choice.
Patients who are selected for CDT should be started on aspirin and heparin as soon as possible followed by angiography and placement within the occluded vessel of an infusion catheter with multiple side holes. Thrombolytic agents are introduced through the infusion catheter for a period of 12 to 24 hours, after which angiography is repeated to evaluate results and identify any underlying lesions, which are then usually corrected by PTA with or without stenting (Fig. 111–12). Thrombolytic agents used include streptokinase, urokinase (no longer available commercially in the United States), rt-PA, reteplase, and tenecteplase. Urokinase is the most studied treatment of ALI; however, rt-PA, reteplase, and tenecteplase are now successfully used to treat patients with ALI. Recently, several studies have compared the outcome after use of different thrombolytic agents. Ouriel129 demonstrated similar bleeding complication rates after use of rt-PA and urokinase. However, in-hospital mortality rates were lower in the urokinase-treated group, even though overall hospital costs did not differ significantly in the two groups.129
Figure 111–12.
Abdominal aortograms in a 54-year-old woman with a history of peripheral artery disease treated by aortofemoral bypass presented with acute onset of severe claudication in the right lower extremity. A. Abdominal aortography showed occlusion of the right limb of the graft (arrow). B. After 16 hours of intraarterial administration of a thrombolytic through a perfusion catheter.
In addition to CDT, percutaneous mechanical thrombectomy (PMT) has also been used to treat ALI patients. PMT allows rapid debulking of thrombus burden as a stand-alone thrombectomy therapy or as an adjunct to thrombolytic therapy to decrease symptomatic ischemia time.130 Of the devices developed to disrupt thrombus formation and remove freshly formed thrombus from the circulation, only the AngioJet Rheolytic Thrombectomy System (Possis Medical, Inc., Minneapolis, MN) is currently approved in the United States for use in the arterial circulation. Here, dissolution of the thrombus occurs within an area of continuous mixing referred to as the “hydrodynamic vortex,” where the thrombus is trapped, dissolves, and is finally evacuated. Several studies131,132 have shown this device to be effective in the treatment of ALI, but it is generally used as an adjunct to CDT rather than as stand-alone therapy. Used together, PMT and CDT appear to hasten reperfusion and reduce the dose and duration of thrombolytic infusion, thus achieving successful reperfusion with lower rates of complications. Rheolytic thrombectomy (RT), with or without chemical lysis, can lead to effective thrombus removal in a short time and reduce major adverse events (MAEs) in ALI patients. In a series reported by Allie et al,133 the 30-day limb salvage rate was 91% in 49 ALI patients using the power-pulse spray technique (P-PS) and RT. Shammas and colleagues134 recently studied the presence of thrombus, using intravascular ultrasonography to evaluate the feasibility of combined thrombolysis (P-PS) and RT in patients with ALI and recent-onset (6 months) limb ischemia. They observed that the application of the P-PS/RT led to partial or complete thrombus resolution in about two-thirds of the patients treated, and the overall safety outcome was favorable.
Endovascular Treatment for Critical Limb Ischemia (CLI): American College of Cardiology/American Heart Association Practice Guidelines44
Class I
For individuals with combined inflow and outflow disease with CLI, inflow lesions should be addressed first.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree