Minimally Invasive Techniques for the Management of Lung Cancer
Robert J. McKenna Jr.
The hope for video-assisted thoracic surgery (VATS) treatment of lung cancer is that it would reduce morbidity, mortality, and hospital stay and allow quicker return to regular activities for patients after procedures that formerly required major incisions. However, since the first VATS lobectomy (VL) with anatomic hilar dissection was performed in 1992, some have questioned the safety and benefits reported for a VATS approach. 1,2,3 Although there is no large, randomized prospective study from around the world, the experience is now sufficiently large enough to compare VATS with open thoracotomy for pulmonary resection. Large series of VATS lobectomies show impressive results. More and more, thoracic surgeons offer their patients minimally invasive pulmonary resections. This chapter will provide the current data regarding minimally invasive procedures for the management of lung cancer.
QUESTIONS ABOUT VATS LOBECTOMY
The following are the current questions about VATS for lobectomy:
What is the definition of a VATS lobectomy?
How is it done?
Is it safe?
Is it an adequate cancer operation?
Are there advantages over a thoracotomy?
DEFINITION
There are some controversies about the exact definition of a VL, and these issues include rib spreading, instrumentation, and anatomic dissection. However, most agree that a VL should include an anatomic dissection of pulmonary vessels, a nodal sampling or dissection, an incision ≤10 cm long, and, most importantly, no rib spreading. Visualization should be on a monitor, not through the incision. A retractor to hold open the soft tissue may be helpful to prevent expansion of the lung during intrapleural suctioning. The use of standard, open instruments or disposable, minimally invasive instruments does not matter and should not be part of the definition. Simultaneous ligation of the hilar structures 1 should be discouraged. A “hybrid” operation that involves rib spreading through a small incision has only recently been addressed in the literature, and surely is used by many surgeons as a compromise between complete VATS and open thoracotomy. There are few reports comparing these techniques4 and patients undergoing lobectomy and lymph node dissection with a complete VATS had less blood loss, faster recovery, shorter hospitalization, and longer operating times than did patients undergoing the lobectomy with the open approaches. At a mean follow-up of 38.8 months, Kaplan-Meier probabilities of survival at 5 years were complete VATS, 96.7%; hybrid VATS, 95.2%; and open techniques, 97.2%. There was no significant difference in the rate of recurrence among the three different procedures. It was concluded that VATS lobectomy is an acceptable cancer operation for patients with peripheral non-small cell lung cancer less than or equal to 2 cm in diameter (clinical stage IA) with the same long-term survivals as open surgery.
GENERAL APPROACH FOR A VATS LOBECTOMY
Philosophically, a lobectomy should be the same operation, whether it is performed through a thoracotomy or by VATS. The arteries, veins, and bronchi should all be individually ligated and a lymph node dissection or sampling should be performed.5
With current technology, most lobectomies can be performed by VATS. In 2005, although 94% of the 239 lobectomies performed by our group were VATS, only 18% of lobectomies in the 2005 Society of Thoracic Surgeons General Thoracic Surgery Database were performed by VATS (personal communication). The adoption for VATS lobectomy was slow after the first VATS lobectomy in 1992, but the momentum is currently growing rapidly, because patients demand the procedure and thoracic surgeons gain an understanding of the techniques to perform the procedure.
TABLE 31.1 Indications and Contraindications for VATS Lobectomy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
INDICATIONS AND CONTRAINDICATIONS
The indications for a lobectomy (Table 31.1) are similar whether the procedure is performed by VATS or via a thoracotomy. For VATS, the ideal case is a T1N0 peripheral tumor; however, we have removed an 8-cm tumor through a 5-cm incision by cutting one rib. Centrally located tumors that require a bronchial sleeve resection are generally performed via a thoracotomy, although VATS sleeve resections have been performed with good results.6 VATS may expand the indications to include patients with marginal performance status and who are not candidates for a thoracotomy.
The contraindication and possible contraindications to a VATS lobectomy are also shown in Table 31.1. Safe dissection around the vessels and in the mediastinum after neoadjuvant chemotherapy and/or radiation is challenging and may require a thoracotomy. Reports do show that VATS lobectomies after neoadjuvant chemotherapy, and sometimes after chemotherapy and radiation treatment, can be performed safely.7
TECHNIQUE FOR VATS LOBECTOMY
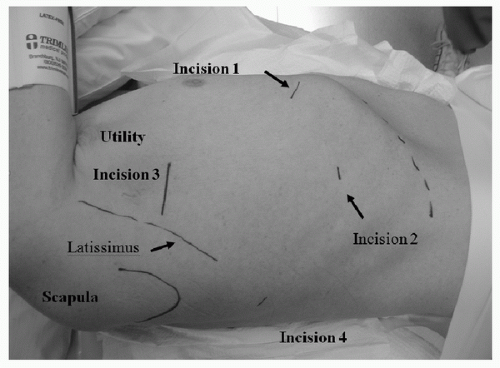
Procedure Typical placement of incisions for a VATS lobectomy is seen in Figure 31.1. The utility incision is 4 to 6 cm, and the ribs are not spread. The fissure, bronchus, and pulmonary vessels larger than 5 mm are individually transected with the endoscopic stapler. Smaller vessels may be tied or clipped. The completeness of the fissure is not a factor in determining the feasibility of performing a VATS lobectomy, because the vessels are stapled anteriorly and then the fissure is completed. Vessels are dissected anteriorly, not through the fissure; the fissure is usually completed after the vessels and bronchus are transected. To minimize the risk of contaminating the incision with the tumor, the lung specimen is placed in a bag for removal through the utility incision.
Lymph Node Dissection Mediastinal node sampling or complete lymph node dissection should be part of all lobectomies for cancer; and they can properly be performed by VATS. In a prospective study, after one surgeon performed a VATS nodal dissection, another thoracic surgeon then performed a thoracotomy to remove any additional lymphatic tissue that could be found.8 For the right-sided procedures, the mean numbers of lymph nodes resected by VATS was 40.3 (weight = 10 g), and mean number of additional nodes found at thoracotomy was 1.2 (weight = 0.2 g). Therefore, a good node dissection can be performed by VATS.
IS A VATS LOBECTOMY SAFE?
Table 31.2 shows the result of several large series. The morbidity and mortality are low in these series, and the length of stay is short. These statistics are comparable or better than those for lobectomy by thoracotomy. The mortality, morbidity, and length of stay for the VATS approach are comparable or better than the results with current series utilizing a thoracotomy. 9 A small randomized study showed fewer complications for the VATS approach (18% vs. 50%).10
Conversion to Thoracotomy Conversion from VATS to thoracotomy is not a failure for a VATS lobectomy. Conversion to thoracotomy was necessary in 0% to 19.5% of patients in large series of VATS lobectomy referenced in this chapter. This was most often required for oncologic reasons, such as a centrally located tumor requiring vascular control,
a sleeve resection, or an unsuspected T3 tumor, attached to the chest wall, diaphragm, or superior vena cava, prompted the conversion. Abnormal hilar nodes with granulomatous or metastatic disease adherent to the superior pulmonary vein may be better evaluated and more safely resected with thoracotomy. Approximately 30% of the conversions to thoracotomy are for nononcologic reasons, such as pleural symphysis.
a sleeve resection, or an unsuspected T3 tumor, attached to the chest wall, diaphragm, or superior vena cava, prompted the conversion. Abnormal hilar nodes with granulomatous or metastatic disease adherent to the superior pulmonary vein may be better evaluated and more safely resected with thoracotomy. Approximately 30% of the conversions to thoracotomy are for nononcologic reasons, such as pleural symphysis.
TABLE 31.2 Results after VATS Lobectomy for Several Large Series | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree