Minimally Invasive Esophageal Procedures
Nikiforos Ballian
Matthew J. Schuchert
James D. Luketich
INTRODUCTION
Minimally invasive surgical approaches are being increasingly implemented in the management of esophageal disease. The continued challenge for the minimally invasive surgeon is to maintain the fundamental principles of esophageal surgery that have been established with decades of open experience, while avoiding ill-advised shortcuts. The more complex the esophageal intervention, the more likely we are to see difficulty in reproducing the key technical steps of any given minimally invasive procedure. In most cases it is not the intent of the surgeon to perform a less than perfect operation, but one may be prodded by reports from other centers, as well as by patients and referring physicians seeking good results from small incisions. Therefore, as we evolve toward a less invasive culture and embrace new technologies, it is important that we continue to critically interpret and publish our results, while adopting a thoughtful attitude toward accomplishing the best outcomes possible.
This chapter summarizes the role of minimally invasive surgery in the treatment of esophageal cancer. Though laparoscopic fundoplication represents one of the cornerstones of minimally invasive esophageal surgery, this topic is discussed in detail in the chapter on antireflux procedures (Chapter 16). Similarly, other advanced minimally invasive esophageal techniques including esophageal staging techniques (Chapter 2), myotomy with fundoplication for esophageal motility disorders (Chapter 20), diverticulectomy (Chapter 22), palliative esophageal procedures (Chapter 21), and paraesophageal hernia repair with or without gastroplasty (Chapter 35) are covered in this text. The minimally invasive approach to esophagectomy highlighted in this chapter includes a detailed description of operative technique as well as a discussion of the published outcomes with comparison to the comparable open procedures. Complex procedures such as minimally invasive esophagectomy (MIE) are routinely being performed in only a limited number of specialized centers. Prospective studies ultimately will be required to objectively identify the benefits and limitations of these minimally invasive approaches.
BACKGROUND—ESOPHAGEAL CANCER
Esophageal cancer is the sixth leading cause of cancer-related death world-wide. In the United States, there will be an estimated 17,460 new cases of esophageal cancer diagnosed in 2012, with over 15,070 deaths (ninth leading cause of cancer-related deaths in the United States). The incidence of esophageal cancer increases steadily with age, with the median age of diagnosis being 68 years. Though squamous cell carcinoma has been demonstrated to be four to five times more common in African Americans, the incidence of adenocarcinoma has been increasing rapidly among Caucasians. In the mid-1990s, adenocarcinoma overtook squamous cell carcinoma as the most common type of esophageal cancer in the United States. Risk factors for the development of adenocarcinoma include chronic gastroesophageal reflux disease (GERD), obesity, and the presence of Barrett’s esophagus. Esophageal adenocarcinoma is currently the solid malignancy with the most rapidly increasing incidence in the United States and the Western world, having increased by almost 600% since the 1970s. Overall survival rates, however, remain grim, ranging between 5% and 16%. The introduction of new molecular diagnostic tests in addition to new and emerging therapeutic techniques, and the development of more effective multimodality management strategies, provide hope for earlier identification and hopefully improved outcomes in the management of this lethal condition.
ENDOSCOPIC MANAGEMENT OF HIGH-GRADE DYSPLASIA AND SUPERFICIAL ESOPHAGEAL CANCER
High-grade dysplasia (HGD), referred to by some as carcinoma in situ, is characterized by malignant histologic findings that are confined to the esophageal epithelium and do not penetrate the basement membrane. HGD is believed to represent a late step in the dysplasia-carcinoma sequence, and can exist both as an isolated lesion and in association with invasive cancer. Although not all patients with HGD have, or will develop, cancer, malignant progression has been documented in 16% to 59% of patients followed for more than 5 years. Detection is notoriously difficult due to the lack of visible mucosal changes, and diagnosis is complicated by significant interobserver variability in the interpretation of the pathologic specimens. Esophagoscopy employing the Seattle protocol (four-quadrant jumbo biopsies taken every 1 cm) remains the standard method for evaluating segments of Barrett’s esophagus. When HGD is suspected based upon pathologic evaluation, confirmation of the diagnosis by an independent pathologist is performed.
The principal management options for patients diagnosed with HGD include endoscopic surveillance, mucosal ablation, or esophagectomy. Intensive endoscopic surveillance can be performed every 3 to 6 months in the setting of HGD, with surgical or ablative therapy being employed once there is definitive evidence of adenocarcinoma. Although it is true that not all patients with HGD will go on to develop cancer, by the time biopsy specimens reveal HGD, between 40% and 60% patients will already have an invasive malignancy. Intensive surveillance strategies might avoid the risks associated with esophageal resection, but there has not been a clear demonstration that current intensive surveillance protocols are cost-effective or improve
mortality from esophageal cancer. Extensive work is underway evaluating methods to enhance the yield of surveillance protocols utilizing improved imaging modalities (e.g., fluorescence endoscopy, narrow-band imaging). However, at this time, none of these modalities has been clearly established as the standard of care in the management of HGD and early esophageal cancer.
mortality from esophageal cancer. Extensive work is underway evaluating methods to enhance the yield of surveillance protocols utilizing improved imaging modalities (e.g., fluorescence endoscopy, narrow-band imaging). However, at this time, none of these modalities has been clearly established as the standard of care in the management of HGD and early esophageal cancer.
Endoscopic ablation represents another option in the treatment of HGD or carcinoma in situ. The intent of this procedure is to eradicate the abnormal Barrett’s epithelium to allow for subsequent restoration of normal squamous mucosa. Multiple techniques have been developed to selectively eradicate Barrett’s mucosa and intramucosal carcinomas, including photodynamic therapy, endoscopic mucosal resection (EMR), and radiofrequency ablation (RFA).
Photodynamic Therapy—Technique
Photodynamic therapy (PDT) employs a photosensitizing drug (porfimer sodium) that is absorbed and retained at higher concentrations in neoplastic tissue when compared with normal tissue. Porfimer sodium (Axcan Scandipharm, Inc., Birmingham, AL) is injected at a dose of 2 mg/kg, and is allowed to circulate for 24 to 48 hours prior to treatment. Endoscopy is performed, and a PDT probe of appropriate length (1 cm, 2.5 cm, or 5 cm) is chosen and deployed through the endoscope under direct visual guidance. The probe is typically deployed in the stomach, pulled back to the designated site of treatment, and maintained in immediate proximity to the targeted lesion throughout the course of therapy. Focal stimulation of porfimer sodium in vivo with a laser light of appropriate wavelength (630 nm) induces a photochemical reaction that results in mucosal destruction. Total light dose ranges between 175 and 300 J/cm. After the initial application is completed, a second look is performed typically within 48 hours to permit assessment of treatment response, perform endoscopic mucosal debridement, and to apply a second dose of PDT, if needed. Patients are maintained on a liquid diet between PDT treatments advancing to a soft diet in 2 to 3 days following the completion of therapy.
Photodynamic Therapy—Results
The endoscopic ablation of Barrett’s esophagus and superficial cancers with PDT has been evaluated in several prospective studies. Complete ablation of HGD or carcinoma in situ can be accomplished in 50% to 77% of cases, with complete squamous reepithelialization occurring in about half. Residual subsquamous intestinal metaplasia has been identified in 5% to 50% of patients after PDT, raising concerns regarding the possibility of continued malignant progression. The most common complication following PDT is stricture formation, occurring in one-third to half of the patients. Other complications following PDT include cutaneous photosensitivity, chest pain, nausea, pleural effusions, Candida esophagitis, atrial fibrillation, and odynophagia. Although more severe complications such as esophageal perforation and tracheoesophageal fistula formation have been reported, they are exceedingly rare (less than 1% in most large studies). Progression to cancer following PDT for HGD has been reported in 5% to 13% of patients. Our experience at the University of Pittsburgh included 50 high-risk patients with either HGD or localized esophageal cancer. At a mean follow-up of 28.1 months, the intent-to-treat success rate was 38% in HGD and 30% in cases of focal carcinoma. Strictures occurred in 42% of patients.
Endoscopic Mucosal Resection— Technique
EMR is also a viable treatment option in patients with nodular or focal HGD or early esophageal cancer. This group of patients has a lower risk of lymph node metastasis, and thus local resection techniques can be performed with the intent to cure. Advantages of EMR also include the generation of a tissue specimen that provides information on stage and margins and lower morbidity and mortality when compared with esophageal resection.
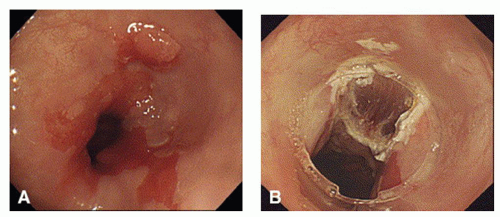
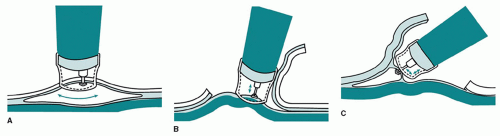
At endoscopy, the targeted lesion must be carefully assessed to determine the extent of resection. Visual delineation of the borders of the lesion can be enhanced by the use of chromoendoscopy or narrow-band imaging. Endoscopic ultrasound (EUS) is also frequently utilized to assess lesion depth and the status of the periesophageal lymph nodes. The EMR technique involves raising the mucosal or submucosal target area by intramural saline and/or suction and then performing a snare resection (Fig. 19.1). This technique can be performed focally or circumferentially. The most common EMR technique utilized in the esophagus is the “cup and suction” or EMR-cap technique. Following injection of saline (with or without epinephrine or methylene blue) into the submucosa to elevate the targeted lesion, a plastic cap is then positioned over the targeted site and the mucosa is drawn into the cap by suction. A snare that is positioned around the cap is then closed around the base of the lesion, which is then removed by electrocautery (Fig. 19.2).
Another commonly employed EMR technique is band ligation. The targeted lesion is suctioned into a ligation cylinder (Duette System, Cook Medical, Winston-Salem, NC) and a band is deployed at the base, which results in the formation of a pseudopolyp. A snare is deployed just below the base of the pseudopolyp to excise the lesion. Other EMR techniques include the strip biopsy, which is most useful for raised or polypoid lesions. No submucosal injection is performed with this technique.
A snare is introduced through the working channel and the targeted lesion is removed by electrocautery. A variation of the strip biopsy is the “inject, lift, and cut” technique, which requires a two-channel endoscope. Following submucosal injection, the lesion is lifted by forceps introduced through one channel, and removed by snare electrocautery performed via the second channel.
A snare is introduced through the working channel and the targeted lesion is removed by electrocautery. A variation of the strip biopsy is the “inject, lift, and cut” technique, which requires a two-channel endoscope. Following submucosal injection, the lesion is lifted by forceps introduced through one channel, and removed by snare electrocautery performed via the second channel.
After resection, the specimen is oriented and submitted to pathology for assessment of pathologic T stage, surgical margins, and for the presence of lymphovascular invasion. For lesions with negative margins and no lymphovascular invasion the probability of a curative resection is >95%. For lesions where margin status remains in question, close endoscopic follow-up is warranted to rule out the possibility of local recurrence. If there is suspicion of lymphovascular invasion or nodal involvement, standard surgical resection with nodal dissection is recommended.
Endoscopic Mucosal Resection— Results
In properly selected patients, EMR can achieve complete remission in over 95% of cases, with corresponding estimated 5-year survival rates as high as 98%. When local recurrences are encountered, they can almost always be treated with by repeat EMR. EMR is now being performed with high success, both focally and circumferentially, for HGD and superficial cancers. Bleeding is the most common minor complication. Esophageal stricture is a late complication and is reported in up to 30% of cases, especially when EMR is performed circumferentially or when combined with other ablative modalities such as PDT. Risk of esophageal perforation is less than 1%.
In conclusion, EMR is a viable technique for the management of HGD and early esophageal cancer. It can be labor intensive and time consuming, especially for larger lesions. Large lesions typically require piecemeal excision, which can complicate assessment of surgical margins. Further randomized studies with long-term follow-up will be required to fully delineate the potential benefits of this approach.
Endoscopic Submucosal Dissection—Technique
Endoscopic submucosal dissection (ESD) represents an extension of the EMR technique and employs an electrocautery knife to dissect and excise larger (>2 cm) mucosal lesions in an en bloc fashion. Similar to EMR, the borders of the lesion are delineated by scoring the mucosa with electrocautery. A saline-epinephrine solution is then injected in the submucosal plane. Other injectable solutions (e.g., hyaluronic acid) can be utilized to maintain the lifting effect on the mucosa, which is then excised utilizing an endoscopic electrocautery knife. A peripheral incision is performed to initiate the dissection. Submucosal fibers are sequentially hooked and cut, and hemostasis is maintained by the use of electrocautery (Fig. 19.3). A transparent cap can be utilized on the tip of the endoscope to enhance exposure. A variety of endoscopic cutting knives has been utilized as reported in the literature, including the Needle Knife (Olympus, Tokyo, Japan), Hook Knife (Olympus, Tokyo, Japan), Flex Knife (Olympus, Tokyo, Japan), Triangle Tip Knife (Olympus, Tokyo, Japan), IT Knife 2 (Olympus, Tokyo, Japan), Duaknife (Olympus, Tokyo, Japan), Safe Knife (Pentax Corp., Tokyo, Japan), Flush Knife (Fujinon Corp, Omiya, Japan), and Hybrid Knife (Erbe Corp, Tübingen, Germany).
Endoscopic Submucosal Dissection—Results
Similar to EMR, successful en bloc resection achieving negative margins has been reported in 95% to 100% of cases with the ESD technique, with recurrence reported in <3% of cases in small series. Complications of ESD include bleeding, perforation, stricture, and pain. Bleeding is the most common complication (7% to 11%), and can be managed with electrocautery and applications of topical epinephrine. Specialized endograspers (Cograsper, Olympus, Tokyo, Japan) are particularly useful in applying electrocautery to areas of bleeding during ESD. Perforation rates, as reported, range from 0% to 1%. Stricture is most common in cases that involve resection of greater than one-half of the esophageal circumference, and is managed with periodic esophageal dilation.
A recent report from a multiinstitutional collaborative in Europe (N = 27 esophageal cases) demonstrated successful en bloc resection in only 77.1% of cases. Morbidity rates were 18.5%, including three cases of perforation (11.1%) and a postprocedural bleeding rate of 7.4%. These data indicate that significant additional experience must be gained with this technique to confirm the promising initial experience reported from Japan. Furthermore, long-term results and survival data are lacking for the ESD technique and this approach will need to be validated by prospective, randomized trials prior to more widely applied clinical application.
Radiofrequency Ablation— Technique
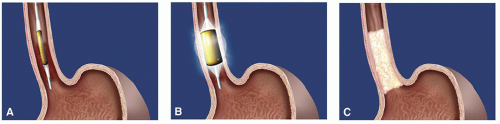
The RFA system (Halo360 System, BARRX Medical Corporation, Inc., Sunnyvale, CA) is a balloon-based catheter ablation system that applies radiofrequency energy via a 3 cm bipolar microelectrode with 60 electrode rings. Radiofrequency energy is delivered through the balloon, generating heat which leads to coagulative necrosis and tissue destruction in the adjacent tissue. A sizing balloon is initially deployed to measure the inner diameter of the targeted segment. The appropriately sized balloon delivery system is then advanced into position over a wire and RFA is performed per a standardized protocol. The closely spaced circumferential electrodes deliver the radiofrequency energy pulse in less than 1 second, accomplishing a uniform distribution of energy density. The depth of ablation is typically limited to the muscularis mucosa. This is slightly more superficial than the EMR and ESD techniques, which excise tissue down to the level of the submucosa. As a result, RFA has been thought to produce a lower rate of stricture formation. The catheter can be repositioned to treat the entire length of Barrett’s esophagus as required (Fig. 19.4). Noncircumferential probes (Halo90 System, BARRX Medical Corporation, Inc., Sunnyvale, CA) that can apply energy to focal islands of residual Barrett’s tissue are available.
Radiofrequency Ablation—Results
In a study of 70 patients with Barrett’s esophagus with no dysplasia treated with RFA (12-month follow-up), metaplasia was completely eradicated in 70% of patients, and partially eliminated in 25% of patients. Adverse events were rare and included chest discomfort and fever. No strictures or residual subsquamous metaplasia were noted. In a multicenter U.S. registry report on 142 patients with HGD treated with RFA, complete resolution was accomplished in 90.2% of patients with HGD, 80.4% with dysplasia, and 54.3% of patients with intestinal metaplasia.
In a multicenter, sham-controlled study evaluating the use of RFA (N = 84) for Barrett’s esophagus with dysplasia, RFA produced complete eradication of dysplastic Barrett’s esophagus in 77.4% of patients, as compared to 2.3% among controls (P < 0.001). Patients in the RFA group had a reduced rate of dysplastic progression (3.6% vs. 16.3%; P = 0.03) during follow-up and a lower incidence of cancer (1.2% vs. 9.3%; P = 0.045). Bleeding occurred in one patient and the esophageal stricture rate was 6.0%. On long-term follow-up (mean follow-up = 3 years), RFA was associated with eradication of dysplasia in >85% of patients, with no evidence of intestinal metaplasia
in >75%. The rate of neoplastic progression was 1 in 73 patient-years, and the rate of progression to carcinoma was 1 in 181 patient-years.
in >75%. The rate of neoplastic progression was 1 in 73 patient-years, and the rate of progression to carcinoma was 1 in 181 patient-years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree