INDICATIONS/CONTRAINDICATIONS
Indications
Tracheal defects may result from surgical resection of primary tracheal tumors or secondary tumors involving the trachea, congenital anomalies such as tracheal atresia, traumatic defects, and trachea malacia or strictures from prolonged intubation. Tumors of the trachea can be primary or secondary. In our practice, the most common tracheal lesions are the result of advanced thyroid cancer involving the trachea.
Small windows of the trachea can be patched or closed primarily. Short tracheal defects may be closed primarily with or without mobilizing the right hilum and laryngeal release. It is generally accepted that a tracheal defect longer than 5.5 cm may not be closed primarily and therefore reconstruction with a tracheal conduit will be required. In cases where patients have had either previous neck surgery or irradiation or are elderly, even a 4–5 cm defect may not be able to be closed primarily due to tracheal calcification, fibrosis and reduced elasticity and blood supply. Therefore, any long tracheal defects that cannot be safely closed primarily are indications for microvascular tracheal reconstruction.
The ultimate goal of tracheal reconstruction is to provide a noncollapsible airway, with a stable epithelial lining and reliable, well-vascularized tissue coverage. Our preferred approach is to use a vascularized radial forearm fasciocutaneous flap for epithelial lining and a prosthetic material for rigid support. A well-vascularized muscle flap may also be necessary to provide coverage to protect the neotrachea as well as great vessels. This is particularly important when postoperative radiotherapy is planned.
Contraindications
Contraindications for complicated tracheal reconstruction can be systemic or local. Severe systemic comorbidities such as major cardiovascular and pulmonary diseases may result in life-threatening complications. Thus, complex reconstruction should be avoided in these patients who may be a prohibitive risk. Locally, if the larynx is already compromised as a result of a radiation stricture or if the resection margin is too close to the vocal cords, preservation of the larynx may result in a poor functional result. Placing sutures through a calcified thyroid cartilage is extremely difficult and may increase the risk of an air leak or dehiscence. The presence of unilateral vocal cord paralysis in these patients may cause further respiratory decompensation. These patients may be better served with a total laryngectomy. It should be pointed out; however, that unilateral vocal cord paralysis alone is not a contraindication to this procedure.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Tracheal reconstruction requires careful planning and a team effort with experts from many disciplines. These include head and neck surgery, thoracic and cardiovascular surgery, reconstructive surgery, critical care, and anesthesiology, as well as specialty nursing care for the early recognition and the avoidance of life-threatening complications. Anesthesiologists must have experience in airway surgery and a good basic understanding of the sequence of tracheal reconstruction and be prepared to frequently change the endotracheal tube during surgery. Surgeons from different services, including reconstructive surgery, must be familiar with airway management as well as bronchoscopy techniques. Many patients have unilateral vocal cord paralysis, which further complicates surgery by increasing the risk for aspiration and airway compromise. Major tracheal surgery and reconstruction thus should be performed in specialized centers with expertise from multiple disciplines.
A thorough evaluation of the extent of disease involvement or defect of the trachea should be performed with imaging studies and bronchoscopy. The status of the remaining airway and lung functions should also be evaluated. For lesions in the cervical trachea, disease involvement of the larynx and vocal cord function are carefully assessed. A history of external beam radiation greatly increases the degree of difficulty in the surgical dissection and the surgical risks. The field of radiation should be obtained for reconstructive planning. A history of neck dissection, especially a combination of neck dissection and external beam radiation, may result in the lack of recipient vessels for microvascular reconstruction and pose significant risk for major blood vessel blowouts during surgical dissection of the trachea and recipient vessels. Vascular status in the neck and upper chest should be evaluated with CT angiography (CTA).
The radial forearm donor site needs to be carefully evaluated for history of trauma, hand dominance, and vascular dominance. The forearm on the side of the patient’s nondominant hand is chosen. An Allen test is commonly performed to assess the integrity of the palmar arches although its reliability is questionable. A normal Allen test usually indicates an intact palmar arch and the radial forearm flap can be safely harvested. In patients with an abnormal Allen test, the radial forearm flap can still be safely harvested in most patients. However, radial artery reconstruction with a saphenous vein graft should be prepared in case the hand perfusion is compromised after harvesting the flap. Once the side of the radial forearm flap is decided in the outpatient setting, patients are advised not to have blood drawn or intravenous lines or arterial lines placed in that arm prior to the surgical date.
The availability of prosthetic material for rigid support should be confirmed. A Montgomery T tracheostomy tube should also be available.
 SURGERY
SURGERY
Routine deep vein thrombosis prophylaxis is given according to the risks and guidelines. Prophylactic antibiotics are also given before making an incision and redosed accordingly.
Positioning
If thoracotomy is not required, patients are placed in a supine position. The head, neck, and chest are surgically prepared. The nondominant forearm is also prepared, wrapped with sterile sheets, and secured on the abdomen. The ablative surgeons perform the resection of the tracheal pathology and the tracheal defect is created. At this point we harvest the radial forearm flap. The arm is placed on an arm board taking care not to extend the arm beyond 90 degrees. Harvesting the radial forearm flap is usually performed in a sitting position.
Techniques
Evaluation of the Defect
Once it is determined that primary end to end anastomosis is not possible, free flap reconstruction is planned. The length and width (circumference) of the defect is measured. The proximal extent toward the larynx and the distal extent toward the carina are assessed (Fig. 43.1).
Preparation of Recipient Vessels
For most tracheal defects, reconstruction can be performed through the neck incision with or without removing the clavicular heads and manubrium. Recipient vessels for microvascular anastomosis are usually readily available in the neck. The superior thyroid artery and a branch of the common facial vein stump that drains into the internal jugular vein are commonly used as recipient vessels. Alternatively, the transverse cervical artery and vein lower in the neck can be used. The third option is the internal mammary artery and vein if the clavicular head and manubrium are removed. The distal part of the recipient artery and vein are clipped or ligated. The proximal stump of the recipient vessels are occluded with a Biover microvascular clamp or Acland vascular clamp with a clamping force of 20 to 30 g/mm2 before dividing the vessels. The clamping and division of the recipient vessels can also be performed after the radial forearm flap is harvested to minimize clamp time. The arterial inflow is checked by releasing the clamp to ensure that pulsatile flow is present.
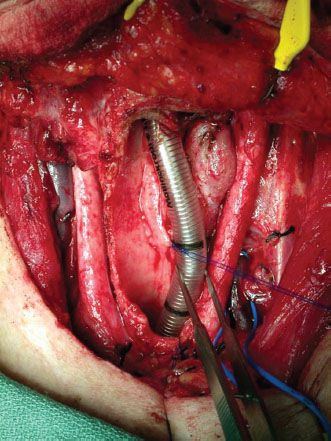
Figure 43.1 A tracheal defect following resection of a recurrent papillary thyroid cancer. The defect extends from the thyroid cartilage to 5 cm above the carina and involves three quarters of the circumference.

Figure 43.2 The PolyMax mesh is placed in a water bath with the water temperature at 70°C. Once it becomes soft, a tubular structure can be formed over a large syringe with a diameter of 30 mm.
Preparation of the Prosthetic Material
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


