High-risk percutaneous coronary intervention (PCI) is often offered to patients with extensive coronary artery disease, decreased left ventricular function, and co-morbid conditions that increase surgical risk. In these settings, percutaneous left ventricular assist devices (PVADs) can be used for hemodynamic support. To assess the effects of PVAD use on mortality, myocardial infarction, and complication rates in patients undergoing high-risk PCI, we systematically searched the electronic databases, MEDLINE, PUBMED, EMBASE, and Cochrane for prospective controlled trials and cohort studies of patients that received hemodynamic support with PVADs for high-risk PCI. The primary outcome measures were 30-day all-cause mortality, 30-day myocardial infarction rates, periprocedural major bleeding, and vascular complications. We included 12 studies with 1,346 participants who underwent Impella 2.5 L device placement and 8 cohort studies with 205 patients that received TandemHeart device for high-risk PCI. Short-term mortality rates were 3.5% and 8% and major bleeding rates were 7.1% and 3.6% with Impella and TandemHeart, respectively. Both devices are associated with comparable periprocedural outcomes in patients undergoing high-risk PCI.
Patients with left ventricular (LV) dysfunction who undergo percutaneous coronary intervention (PCI) for complex coronary artery disease (CAD) such as multivessel, bypass graft, or left main CAD are at increased risk of periprocedural hemodynamic compromise and cardiovascular complications. Although the intraaortic balloon pump (IABP) is the most commonly used mechanical support device, it only provides modest hemodynamic support, and its use is not associated with survival benefit in the setting of high-risk PCI or cardiogenic shock. Percutaneous left ventricular assist devices (PVADs) can be expeditiously inserted under fluoroscopy in the cardiac catheterization laboratory and provide partial or complete hemodynamic support during high-risk PCI by reducing LV volumes, wall stress, and myocardial oxygen consumption and augmenting cardiac output and coronary perfusion. We performed a systematic review and meta-analysis to assess the effects of PVAD use (Impella-Abiomed Inc., Danvers, Mass axial flow left ventricle assist device and Tandem-Heart-CardiacAssist, Pittsburgh, Pennsylvania centrifugal pump) on mortality, MI, and complication rates in patients undergoing high-risk PCI.
Methods
Search strategy
Systematic electronic search was performed on MEDLINE, EMBASE, and CENTRAL with no language limitations. We searched with the Medical Subject Headings (MESH) terms “percutaneous left ventricular assist devices,” “Impella” or “TandemHeart,” and “percutaneous coronary intervention”. Two reviewers (AB and TT) independently screened titles and abstracts based on inclusion and exclusion criteria. After eliminating irrelevant studies, full-text reports were reviewed. Studies using surgically implanted assist devices and extracorporeal membrane oxygenation were excluded. Subsequently, we performed hand search of all included studies until no further relevant studies were identified. A total of 20 studies were identified.
Study selection
Out of studies identified, one study was prospective controlled clinical trial and 18 studies were nonrandomized observational cohort studies and registries. We performed a pooled meta-analysis of studies that used Impella or TandemHeart in high-risk PCI. We excluded case reports and case series as well as studies with <10 cases of PVAD placement due to concerns about low operator volume.
Outcomes assessed
The primary outcome measures were 30-day all-cause mortality, 30-day myocardial infarction (MI), periprocedural major bleeding, and vascular complications. Bleeding outcomes were not assessed objectively with the use of semiquantitative scores in most of the studies ( Table 1 ). Therefore, we included all cases that required blood transfusions or were reported to have major bleeding according to the individual study criteria. Vascular complications included (1) access site or access-related vascular injury (dissection, stenosis, perforation, rupture, arteriovenous fistula, pseudoaneurysm, hematoma, irreversible nerve injury, or compartment syndrome) requiring blood transfusions or surgical intervention; (2) distal embolization and limb ischemia; or (3) failure of percutaneous access site closure requiring intravascular or surgical correction.
| Study | Device | Design ∗ | Age (years) | Sample size | Intervention | LVEF | Major bleeding definition |
|---|---|---|---|---|---|---|---|
| Nascimbene et al 2015 | TandemHeart | Cohort | 71 | 33 | Multivessel, LM, bypass grafts | 25% | Requiring more than 2 units of PRBCs |
| Alli et al 2012 | TandemHeart | Cohort | 72 | 54 | Multivessel, LM, bypass grafts | 20% | Requiring transfusion |
| Schwartz et al 2011 | TandemHeart | Cohort | 75.4 | 50 | Multivessel, LM, bypass grafts | 31% | TIMI |
| Kovacic et al 2013 | TandemHeart | Cohort | 71.1 | 68 | Multivessel, LM, bypass grafts | 31% | Requiring transfusion or surgery |
| Vranckx et al 2008 | TandemHeart | Cohort | 65 | 9 | LM | N/A | Requiring transfusion or surgery |
| Rajdev et al 2008 | TandemHeart | Cohort | 70 | 20 | Multivessel, LM | 38% | Requiring transfusion or surgery |
| Kar et al 2006 | TandemHeart | Cohort | 78 | 7 | Multivessel, LM, bypass grafts | N/A | N/A |
| Gimelli et al 2008 | TandemHeart | Cohort | 73 | 11 | Multivessel, LM, bypass grafts | 25% | Requiring transfusion |
| EuroPella 2009 | Impella | Registry | 71.8 | 144 | Multivessel, LM, bypass grafts | 64% had <40% | Requiring transfusion or surgery |
| USPella 2015 | Impella | Registry | 70 | 637 | Multivessel, LM, bypass grafts | 30.1% | Requiring transfusion or surgery |
| Ferreiro et al 2010 | Impella | Registry | 68.2 | 27 | Multivessel, LM, bypass grafts | 33% | TIMI |
| Henriques et al 2006 | Impella | Cohort | >60 | 19 | Multivessel, LM, bypass grafts | <40% | Requiring transfusion or surgery |
| PROTECT I 2009 | Impella | Registry | 60 | 20 | Multivessel, LM, bypass grafts | <35% | Requiring transfusion or surgery |
| Venugopal et al 2015 | Impella | Cohort | 72 | 49 | Multivessel, LM, bypass grafts | 28% | Requiring transfusion or surgery |
| Burzotta et al 2008 | Impella | Cohort | 60.5 | 10 | Multivessel, LM, bypass grafts | 31% | Requiring transfusion or surgery |
| Iliodromitis et al 2011 | Impella | Cohort | 69.7 | 38 | Multivessel, LM, bypass grafts | <45% | Requiring transfusion or surgery |
| Schreiber et al 2016 | Impella | Cohort | 70 | 141 | Unprotected LM | 35% | Requiring transfusion or surgery |
| Boudoulas et al 2012 | Impella | Cohort | 62.5 | 13 | Multivessel, LM, bypass grafts | 24% | Requiring transfusion or surgery |
| Kovacic et al 2013 | Impella | Cohort | 71.9 | 36 | Multivessel, LM, bypass grafts | 26.9% | Requiring transfusion |
| PROTECT II 2012 | Impella | RCT | 67.4 | 216 | Multivessel, LM, bypass grafts | 23.4% | Requiring transfusion |
Risk of bias
Cochrane’s risk of bias tool has been used to assess the individual risk of bias of each prospective randomized study. The Newcastle-Ottawa tool was used for the quality assessment of cohort studies. Two investigators (AB and TT) independently assessed the risk of bias and quality of studies in each eligible trial. Low-quality studies had 2 or more quality assessment criteria qualified as high or unclear risk of bias.
Data analysis, summary measures, and synthesis of results
Systematic review and meta-analysis was done in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Meta-analyses were performed using the Review Manager (RevMan), version 5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, 2012, Copenhagen, Denmark). The chi-square test of heterogeneity and I 2 statistic of inconsistency were used to assess the heterogeneity between studies. I 2 values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity respectively. Pooled effect of intervention was measured using odds ratio with 95% CI. In addition, we used software (StatsDirect version 2.7.9) to calculate the pool estimates of all-cause mortality, MI, major bleeding, and vascular complications, using both fixed- and random-effects models for combining proportions. The Freeman–Tukey variant of the arcsine square root transformation was used to account for the fact that proportions with extreme values have lower variances. A DerSimonian–Laird random-effects model for odds ratios estimation of all outcomes was used in the presence of heterogeneity. Reported values are 2-tailed, and hypothesis-testing results were considered statistically significant at p <0.05. The small study effect, including publication bias, was tested using funnel plot and the Egger’s test.
Results
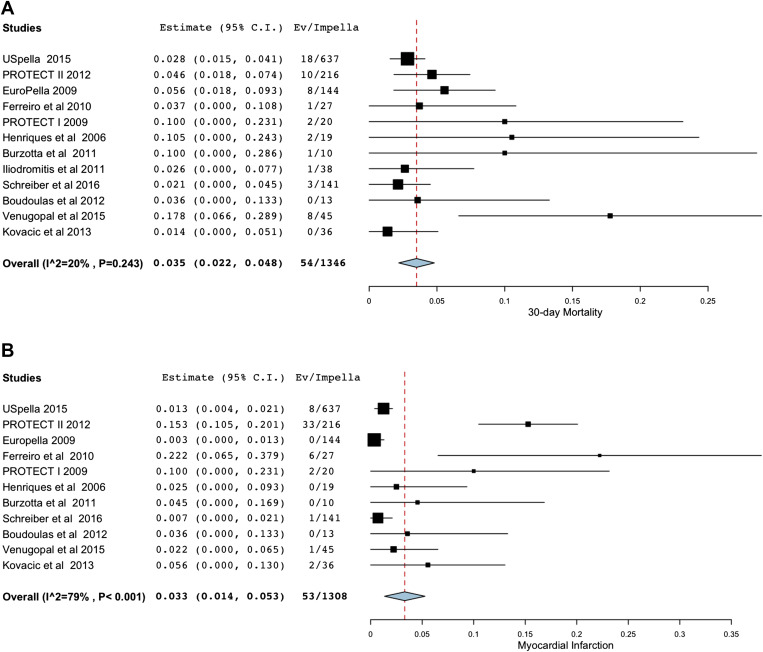
We included 12 studies (11 cohort studies and the Impella arm of the Prospective Randomized Clinical Trial of Hemodynamic Support with Impella 2.5 versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention [PROTECT] II trial ) with a total number of 1,346 participants who underwent Impella 2.5 L device placement for high-risk PCI. We also included and analyzed separately 8 cohort studies with 205 patients who underwent high-risk PCI with hemodynamic support by TandemHeart device. The characteristics of each study are presented in Table 1 . On the basis of quality assessment, the prospective randomized study included in our analysis was deemed to be at low risk of bias. Five cohort studies that were included in the analysis were deemed to be at high risk of bias, whereas the remaining cohort studies were deemed to be at low risk.
Impella for high-risk PCI
The use of Impella in high-risk PCI was associated with pooled clinical 30-day mortality of 3.5% (95% CI 2.2% to 4.8%) without significant heterogeneity between studies (I 2 20%; Figure 1 ). The pooled 30-day MI rate was 3.3% (1.4%, 5.3%) with significant heterogeneity between studies (I 2 79%; Figure 1 ) which became nonsignificant with subgroup analysis without the PROTECT II study. The variability in MI rates maybe due to differences in the definition of MI used in various cohorts, operator experience in different centers, and reporting of MI in cohort studies as opposed to the randomized PROTECT II trial (in which biomarker assessment may have been more frequent compared with cohort studies). The pooled clinical major bleeding rate was 7.1% (4.3%, 9.9%) with significant heterogeneity between studies (I 2 63%) mainly explained by the higher bleeding rates in the USPella registry and PROTECT II study ( Figure 1 ). Finally, the pooled vascular complication rate was 4.9% (2.3%, 7.6%) with significant heterogeneity between studies (I 2 78%, Figure 1 ) mainly due to various definitions of vascular complications.