Transcatheter aortic valve replacement (TAVR) is a viable option in the treatment of severe aortic stenosis in patients at high risk for surgery. We sought to further investigate outcomes in patients at low to intermediate risk with aortic stenosis who underwent surgical aortic valve replacement (SAVR) versus TAVR. We systematically searched the electronic databases, MEDLINE, PubMed, EMBASE, and Cochrane for prospective cohort studies of the effects of TAVR versus SAVR on clinical outcomes (30-day mortality, all-cause mortality, stroke and myocardial infarction, major vascular complications, paravalvular regurgitation, permanent pacemaker implantation, major bleeding, and acute kidney injury). We identified 5 clinical studies, examining 1,618 patients in the TAVR group and 1,581 patients in the SAVR group with an average follow-up of 1.05 years. No difference in all-cause mortality, stroke, and myocardial infarction between the 2 approaches was found. TAVR was associated with higher rates of vascular complications, permanent pacemaker implantation, and moderate or severe paravalvular regurgitation (p <0.001 for all), whereas more major bleeding events were seen in the SAVR group (p <0.001). In conclusion, TAVR was found to have similar survival and stroke rates and lower major bleeding rates as compared with SAVR in patients at low or intermediate surgical risk. However, SAVR was associated with less pacemaker placements and paravalvular regurgitation rates.
Surgical aortic valve replacement (SAVR) has been the principal treatment procedure in operable patients because of improved long-term survival rate. However, decreased long-term survival rate was seen in patients at high surgical risk who underwent SAVR. Meta-analysis of observational studies and randomized clinical trial including the Placement of AoRtic TraNscathetER Valves (PARTNER) trial showed that the transcatheter aortic valve replacement (TAVR) is a preferable alternative in patients at high surgical risk who cannot undergo SAVR and was found to have similar outcomes. In case of patients at low or intermediate surgical risk, the use TAVR has been studied and has suggested either similar or decreased mortality compared with patients at high surgical risk. The purpose of this systematic review and meta-analysis was to explore the outcomes of TAVR over SAVR in patients at low and intermediate surgical risk.
Methods
Systematic electronic search was performed on MEDLINE, EMBASE, and CENTRAL (Cochrane Central Registry of controlled trials) with no language limitations. We searched for aortic valve stenosis, TAVR, and SAVR using the Medical Subject Headings terms. Finally, a total of 5 studies have been identified. Inclusion criteria for study selection include the randomized clinical trials or prospective observational studies performed in severe aortic valve stenosis patients who are at low or intermediate surgical risk. We considered the surgical risk stratification based on Society of Thoracic Surgeons (STS)–defined risk score for patients who underwent isolated valve surgery; low risk (STS <3%), intermediate risk (STS ≥3% and ≤8%), and high risk (STS >8%). Intervention performed was TAVR and compared with SAVR. We excluded studies that were nonhuman, not randomized, and had high surgical risk patients with STS risk score >8%. We did not restrict eligibility based on study outcomes. The primary outcome measures were 30-day mortality, all-cause mortality, stroke, and myocardial infarction (MI). Secondary outcome measures were major vascular complications, moderate or severe paravalvular regurgitation, permanent pacemaker implantation, major bleeding, and acute kidney injury. Periprocedural complications were defined according to the Valve Academic Research Consortium definitions. Paravalvular regurgitation was quantified angiographically, immediately after the procedure in the Observational Study of Effectiveness of SAVR–TAVI Procedures for Severe Aortic Stenosis Treatment (OBSERVANT) trial. In the Nordic Aortic Valve Intervention (NOTION) A Prospective, Randomised Trial of Transapical Transcatheter Aortic Valve Implantation versus Surgical Aortic Valve Replacement in Operable Elderly Patients with Aortic Stenosis and US Pivotal studies, paravalvular regurgitation was evaluated with echocardiography during the follow-up. Propensity-matched data of patients in SUrgical Replacement and Transcatheter Aortic Valve Implantation trial reported only the survival outcomes such as 30-day mortality and all-cause mortality.
Cochrane’s risk of bias tool has been used to assess the individual risk of bias of each study. The Newcastle-Ottawa tool was used for the quality assessment of prospective cohort studies. Systematic review and meta-analysis was done in compliance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement. Meta-analysis was performed using the Review Manager, version 5.3. In the absence of heterogeneity, pooled estimates of odds ratio (ORs) with their 95% CIs were calculated using the Mantel–Haenszel method. In case of significant heterogeneity between studies, DerSimonian and Laird random effects model was used. Reported values are 2-tailed, and hypothesis-testing results were considered statistically significant at p <0.05. Sensitivity analysis was performed by eliminating each study at a time to assess the influence of any included study on the results. The small study effect, including publication bias, was tested using funnel plot and the Egger’s test.
Results
Electronic search of scientific literature identified 1,548 articles. After deduplication, screening of titles and abstracts, and full text review based on inclusion and exclusion criteria, 5 studies were identified and included in the meta-analysis. The SURTAVI and the OBSERVANT trials are prospective cohort studies with groups match by propensity-score analysis.
The total number of participants included in our meta-analysis was 3,199 with mean age of 80 years and mean follow-up duration of 1.05 years. Total number of participants treated with TAVR was 1,618 (50.6%). The characteristics of each study are presented in Table 1 . TAVR procedures were performed using the self-expanding CoreValve System (Medtronic Inc., Minneapolis, MN) in 60.4% of patients in the OBSERVANT trial and also in the US PIVOTAL, NOTION and SURTAVI trials, whereas the balloon-expandable Edwards SAPIEN XT (Edwards Lifescience, Irvine, CA) was use in the STACCATO trial and in 39.6% of patients in the OBSERVANT trial.
| Study | Study design | Sample Size | Mean age (years) | Mean STS score | Median follow up years | Mean change in aortic valve gradient | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAVR | SAVR | Entire cohort | TAVR | SAVR | TAVR | SAVR | ||||
| NOTION trial (2015) | RCT | 145 | 135 | 280 | 79 | 2.9 | 3.1 | 1 | -34.8 | -32 |
| U.S. pivotal trial (2015) | RCT | 391 | 359 | 750 | 83 | 7.3 | 7.5 | 2 | −39.04 | −35.42 |
| Italian OBSERVANT Study (2015) | POS | 650 | 650 | 1300 | 80 | Low to intermediate risk according to EUROSCORE | 1 | -40.7 | -37.5 | |
| SURTAVI trial (2013) | POS | 405 | 405 | 810 | 80 | STS 3-8% | 1 | |||
| STACCATO trial (2012) | RCT | 34 | 36 | 70 | 81 | 3.1 | 3.4 | 0.25 | -61 | -42 |
The incidence of death within 30 days after the intervention was 4.3% in patients treated with TAVR and 4.4% in patients treated with SAVR. No significant difference was found between the 2 groups in 30-day mortality (OR 0.99, 95% CI 0.71 to 1.39; p = 0.97; I 2 = 1%). No significant heterogeneity was observed between the studies ( Figure 1 ). Sensitivity analysis of trials that used the self-expanding CoreValve System (with the exclusion of the STACCATO trial) did not show any difference in 30-day mortality. The incidence of death at the end of the follow-up period was 15.5% in TAVR group and 16.3% in SAVR group. No significant difference was found between the 2 groups in all-cause mortality (OR 0.92, 95% CI 0.70 to 1.21; p = 0.55; I 2 = 37%) without significant heterogeneity among the studies. Sensitivity analysis of studies that used self-expanding CoreValve System did not reveal any differences in the outcome ( Figure 1 ).
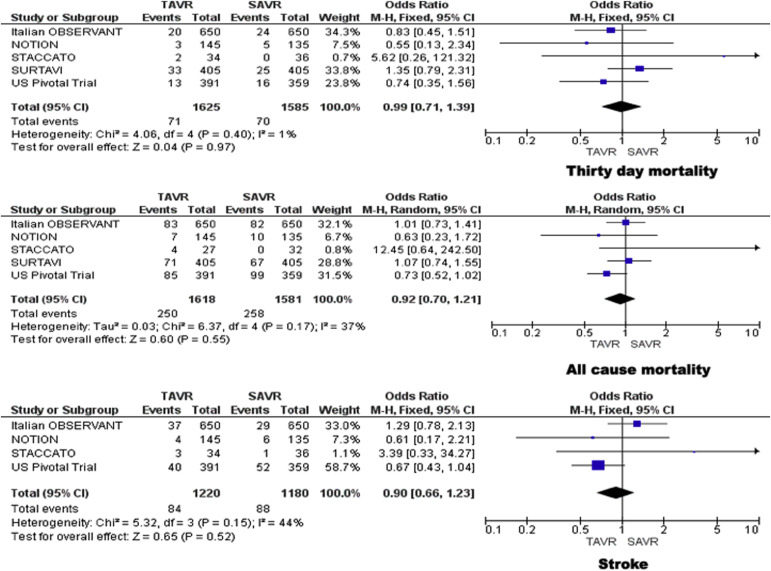
The incidence of stroke was lower for TAVR compared with SAVR (6.9% vs 7.5%). No significant difference was found in incidence of stroke between the 2 groups (OR 0.90, 95% CI 0.66 to 1.23; p = 0.52; I 2 = 44%) without significant heterogeneity among the studies. The results were similar in the subgroup of studies that used self-expanding CoreValve System ( Figure 1 ).
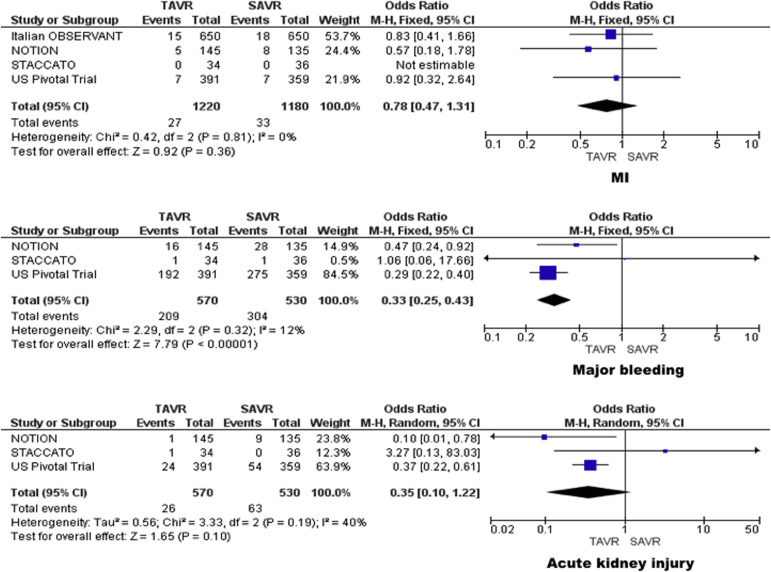
MI was seen in 2.2% of patients treated with TAVR and 2.8% of patients treated with SAVR. No significant difference was found in the incidence of MI between the 2 groups (OR 0.78, 95% CI 0.47 to 1.31; p = 0.36; I 2 = 0%). No significant heterogeneity was observed between the studies. The results did not change in the subgroup of studies that used self-expanding CoreValve System ( Figure 2 ).
The incidence of major vascular complications was 7.0% in patients treated with TAVR and 1.0% in patient treated with SAVR. TAVR was associated with a significant increase in major vascular complications compared with SAVR (OR 7.00, 95% CI 3.81 to 12.87; p <0.001; I 2 = 60%) without significant heterogeneity among the studies ( Figure 3 ).
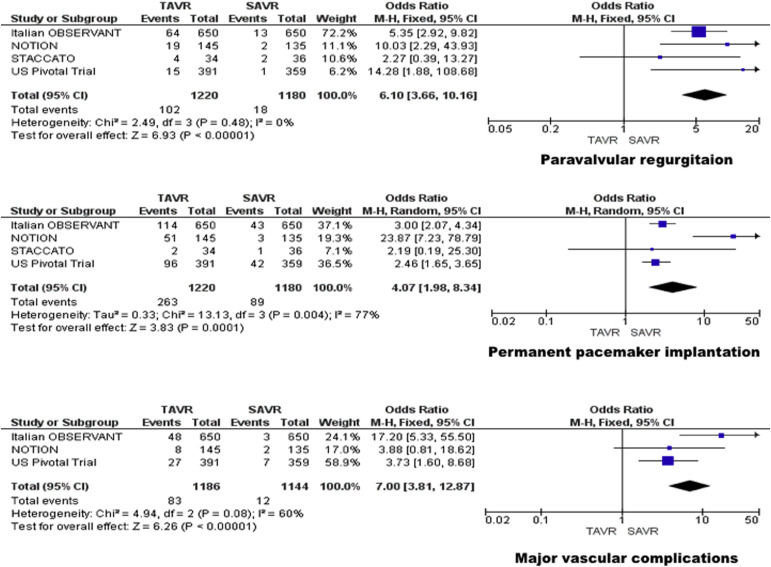
The incidence of moderate or severe paravalvular regurgitation at the end of the follow-up period was 8.4% in patients treated with TAVR and 1.5% in patient treated with SAVR. TAVR group was associated with a significant increase in incidence of moderate or severe paravalvular regurgitation compared with SAVR group (OR 6.10, 95% CI 3.66 to 10.16; p <0.001; I 2 = 0%). Sensitivity analysis of studies, which provided long-term echocardiographic data on paravalvular regurgitation, confirmed the increased incidence in the TAVR group. No significant heterogeneity was observed between the studies ( Figure 3 ).
The patients requiring permanent pacemaker implantation were 21.6% in TAVR group and 7.5% in SAVR group. Permanent pacemaker implantation was significantly higher in TAVR group than SAVR group (OR 4.07, 95% CI 1.98 to 8.34; p <0.001; I 2 = 77%). A significant heterogeneity of was observed which was eliminated after exclusion of the NOTION trial in which pacemaker placement was required in 35% of the patients ( Figure 3 ).
The incidence of major bleeding was 36.7% in patients treated with TAVR and 57.4% in patients treated with SAVR. TAVR was associated with significantly lower major bleeding events compared with SAVR (OR 0.33, 95% CI 0.25 to 0.43; p <0.001; I 2 = 12%). No significant heterogeneity was observed between the studies ( Figure 2 ).
The incidence of acute kidney injury was 4.6% in patients treated with TAVR and 11.9% in patients treated with SAVR. However, no significant difference was found in incidence of acute kidney injury between the 2 groups (OR 0.35, 95% CI 0.10 to 1.22; p = 0.10; I 2 = 40%). No significant heterogeneity was observed between the studies ( Figure 2 ).
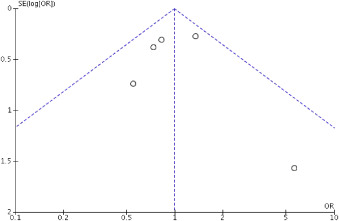
Risk of bias assessment showed low-to-moderate risk of bias in all studies. The funnel plot ( Figure 4 ) did not show asymmetry consistent with publication bias, and the Egger’s test was not significant for the outcomes studied.