Endothelial progenitor cells (EPCs) may concur to endogenous vascular repair. Previous studies have reported that statin treatment increases EPC levels. We investigated whether this occurs in patients on long-term statin treatment who underwent percutaneous coronary interventions (PCIs). In a phase A study, 53 patients (atorvastatin reload [AR] 80 mg 12 hours before + 40 mg 2 hours before PCI, n = 27; placebo [P], n = 26) were evaluated for EPC mobilization as CD45dim/CD34+/CD133+/KDR+ cell number by flow cytometry. Assays were run at randomization (12 hours before PCI, R), immediately before PCI (T0) at 8 (T8) and 24 hours (T24). In phase B study, 50 patients (AR, n = 25; P, n = 25) were evaluated for early colony formation by Hill colony forming unit (CFU) assay, with sampling at randomization and 24 hours later. In phase A, EPCs levels were similar at randomization between 2 arms (0.23% [0.14 to 0.54] of total events in AR vs 0.22% [0.04 to 0.37] in P group; p = 0.33). At PCI, EPC levels were higher in AR arm (0.42% [0.06 to 0.30] vs 0.19% [0.06 to 030]; p = 0.009). Higher EPC levels in AR group were also found at 8 and 24 hours. In phase B, EPC CFUs/well numbers at randomization were similar in the 2 arms (8 [6 to 12] in AR vs 12 [6 to 20] in P group, p = 0.109). EPC CFU/well at 24 hours became significantly higher in AR arm (17 [10 to 23] vs 5 [2 to 13], p = 0.002). In conclusion, high-dose AR before PCI in patients on long-term statin therapy promptly increases EPCs mobilization, which are capable of early colony formation and may contribute to cardioprotection.
Endothelial progenitor cells (EPCs) are a particular population of cells originating from hematopoietic stem cells in the bone marrow, which have been isolated from circulating mononuclear cells in peripheral blood. EPCs may contribute to vascular repair when they are incorporated into sites of physiological and pathologic neorevascularization. The number and function of EPCs are directly related to outcome of patients with coronary artery disease. Previous investigations have suggested that statins promote mobilization of circulating EPCs. Nevertheless, it is still unclear if this effect could contribute to explain benefit of short-term pretreatment with high-dose statin during percutaneous coronary intervention (PCI). Furthermore, whether this may occur in patients already on long-term statin treatment (currently most patients who underwnt PCI) submitted to a high-dose statin reload before PCI is unknown. We designed a randomized study, denominated the Atorvastatin for Reduction of Myocardial Damage During Angioplasty-Endothelial Progenitor Cells (ARMYDA-EPC), with the aims of investigating whether a short-term, high-dose atorvastatin reload is effective in inducing EPC mobilization also in patients on long-term statin treatment who underwent PCI.
Methods
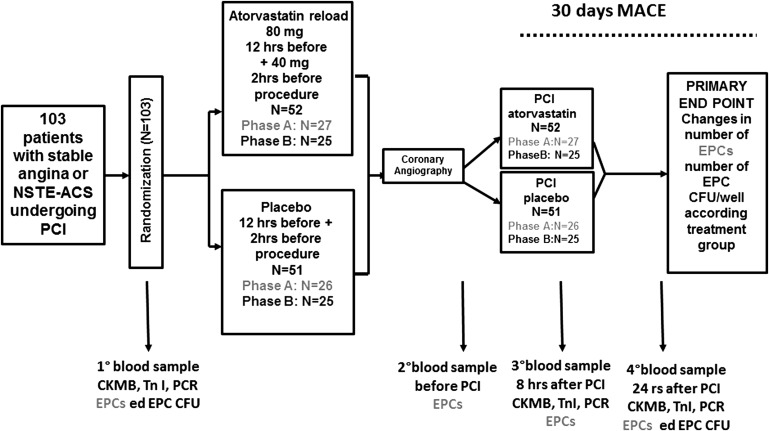
This was a prospective, randomized study performed at Campus Bio-Medico University of Rome in collaboration with the Institute of Cardiology, “G. D’Annunzio” University, Chieti. The design of the study is illustrated in Figure 1 . Patients on long-term (>30 days) statin therapy with stable angina and indication to coronary angiography or non–ST-segment elevation acute coronary syndrome (ACS) requiring invasive strategy were included. Exclusion criteria were ST-segment elevation myocardial infarction, non–ST-segment elevation ACS with high-risk features warranting emergency coronary angiography (<2 hours); baseline increase in liver enzymes (aspartate aminotransferases/alanine aminotransferases); left ventricular ejection fraction <30%; renal failure with a creatinine level >2 mg/dl; history of muscle disease; and history of systemic inflammatory disease or neoplasm. Patients fulfilling inclusion criteria were randomly assigned to receive placebo or atorvastatin (80-mg load given 12 hours before coronary angiography, with a further 40-mg dose 2 hours before the procedure). Patients were assigned to the study arm using an electronic spreadsheet indicating the group assignment by random numbers. The study included 2 phases (A and B). In a first series of patients (phase A), a total of 53 patients (atorvastatin reload, n = 27; placebo, n = 26) were evaluated for EPC mobilization as number of CD45dim/CD34+/CD133+/KDR+ cells, determined by flow cytometry (see Supplementary Figure 1 ). Peripheral blood samples for flow cytometry were drawn at randomization, 12 hours before the procedure (sample R), immediately before PCI (sample T0), and at 8 (sample T8) and 24 hours (sample T24). In a second series (phase B), a total of 50 patients (atorvastatin reload, n = 25; placebo n = 25) were evaluated for early colony formation by the Hill colony forming unit (CFU) assay, expressed as EPC CFU/well (see Supplementary Figure 2 ). Peripheral blood samples for EPC colony assay were obtained at randomization (12 hours before the procedure) and 24 hours later, and processed for culture.
Interventions were performed using a standard technique. All patients were given aspirin (100 mg/day) and received either a 600-mg clopidogrel loading dose (at least 6 hours before PCI) or continued clopidogrel treatment if they were on clopidogrel 75-mg/day therapy for at least 7 days. Procedural success was defined as postprocedure Thrombolysis In Myocardial Infarction grade 3 flow, with decrease of stenosis to <30% residual narrowing by quantitative coronary angiographic analysis. A nonionic, low-osmolarity (915 mOsm/kg) iodinated contrast agent (iobitridol, Xenetix; Guerbet, Roissy CdG Cedex, France) was used. PCIs were performed using the femoral approach after administration of weight-adjusted intravenous unfractionated heparin (70 IU/kg body weight); bivalirudin was used instead of unfractionated heparin in patients considered at high bleeding risk (age >75 years, history of previous bleeding, renal failure, and low body weight). Periprocedural use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. After the procedure, aspirin (100 mg/day) was continued indefinitely, whereas clopidogrel (75 mg/day) was administered for at least 1 month (12 months in patients treated for ACS or receiving a drug eluting stent). All patients received atorvastatin 40 mg/day after PCI, irrespective of the initial randomization assignment. Clinical follow-up was obtained at 30 days by office visits in all patients. Each patient gave written informed consent to the study. Physicians performing the procedure and the assessment of outcome measures were blinded to the randomization assignment. The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Campus Bio-Medico University. The trial was not supported by any external source of funding.
To monitor changes in EPC levels, 3 ml of peripheral blood was collected in EDTA-treated tubes (4 tubes/patient). Blood samples were immediately refrigerated at 4°C until processing within 4 hours of collection. Five hundred microliters of the previously collected blood were mixed with 25-μL dimethyl sulfoxide and cryopreserved in a −80°C freezer for 3 days until flow cytometry. EPCs were identified by flow cytometry as CD45dim/CD34+/CD133+/KDR+ cells, as previously described (further information is provided in the Supplementary Material ). For EPC colony assay, a total of 5 × 10 6 mononuclear cells per well were plated in the 6-well fibronectin-coated plate in duplicate and incubated for 2 days at 37°C, 5% CO2 with 95% humidity (further information on mononuclear cells preparation is provided in the Supplementary Material ). After 2 days, nonadherent cells were collected and plated at 1 × 10 6 cells per well in a 24-well fibronectin-coated plate. Cells were incubated at 37°C, 5% CO2 with 95% humidity for 3 days, then the number of colonies (defined as a central core of round cells with radiating elongated spindle-like cells at the periphery) per well were quantified. Blood samples were also drawn in all patients before PCI and at 8 and 24 hours after PCI for measurement of creatine kinase-MB and troponin I levels. Additional samples were obtained to measure myocardial necrosis markers if patients after PCI developed symptoms suggestive of myocardial ischemia. Measurements were performed using the Access 2 immunochemiluminometric assay (Beckman Coulter, Fullerton, California). Normal limits were set at 3.6 ng/ml for creatine kinase-MB and at 0.06 ng/ml for troponin I.
The primary end point of the study was the evaluation of the effect of atorvastatin reload on both EPC number (phase A) and function (phase B) in patients who underwent PCI. EPC number was expressed as percentage of positive events/total events at flow cytometry (phase A), whereas EPCs functional capacity for early colony formation was expressed as number of EPC CFU/well (phase B) according to randomized treatment. We also evaluate the incidence of periprocedural myocardial damage and major adverse cardiac events (MACE) (cardiac death, myocardial infarction, or unplanned target vessel revascularization) at a 30-day follow-up according to treatment arm. Periprocedural myocardial damage was defined as post-PCI increase in troponin I levels >5 times the 99th percentile of the upper reference limit for patients with normal baseline values or as a post-PCI increase of troponin I values >20% if baseline values were elevated. Periprocedural myocardial infarction was defined according to the Joint European Society of Cardiology/ American College of Cardiology Foundation/American Heart Association/World Heart Federation (ESC/ACCF/AHA/WHF) task force consensus statement on the redefinition of myocardial infarction for clinical trials on coronary intervention.
Results are expressed as mean ± SD unless otherwise specified. Normal distribution was tested by the Kolmogorov–Smirnov test. Continuous variables were compared by 2-tailed t test (paired or unpaired, as appropriate) for normally distributed values; otherwise the Mann–Whitney U test and Wilcoxon signed-rank test were used. Proportions were compared by Fisher’s exact test when the expected frequency was <5 or the Yates-corrected chi-square test otherwise. A p value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 16.0 release software (SPSS, Inc., Chicago, Illinois).
Results
Clinical and procedural features in the active treatment and placebo arms are reported in Tables 1 and 2 . All patients received the study-assigned drug before PCI. No patient had any increase of liver enzymes (alanine aminotransferase or aspartate aminotransferase) above the upper limit of normal value after statin treatment. In phase A, no difference in baseline EPC number was identified between the 2 groups (atorvastatin reload: 0.23% [interquartile range, 0.14 to 0.54] of total events vs placebo: 0.22% [0.04 to 0.37], p = 0.33); EPC number was significantly higher in the reload arm even before PCI (0.42% [0.06 to 0.30] vs 0.19% [0.06 to 030]; p = 0.009); at 8 hours (0.42% [0.11 to 0.34] vs 0.22% [0.11 to 0.34], p = 0.011) and 24 hours (0.44% [0.20 to 0.79] vs 0.23 [0.04 to 0.33]; p = 0.004; Figure 2 ). In the atorvastatin reload arm, a significant increase of EPC levels was observed from baseline to PCI and was persistent in all further measurement after the procedure (EPCs R vs T0, p = 0.009; EPCs R vs T8, p = 0.004; EPCs R vs T24, p = 0.002; Figure 2 ), whereas in the placebo arm, similar EPC levels were found throughout all determinations (EPCs R vs T0, p = 0.93; EPCs R vs T8 p = 0.69; EPCs R vs T24, p = 0.97, Figure 2 ). In phase B, no difference was found between the 2 groups in baseline EPC CFU/well (p = 0.109), whereas at 24 hours after PCI, there was a higher value of EPCs CFU/well in the atorvastatin reload arm versus placebo (p = 0.002; Figure 3 ). After the procedure, a significant increase of EPC CFU/well from baseline levels was observed in atorvastatin reload arm (from 8 [6 to 12] to17 [10 to 23]; p <0.0001), whereas in the placebo arm, EPC CFU/well decreased (from 12 [6 to 20] to 5 [2 to 13]; p = 0.001, Figure 3 ). In the overall population of 103 patients, a numerical reduction of 30 days MACE was observed in patients allocated to the atorvastatin reload arm than in those of the placebo arm (5.8% vs 13.7%, p = 0.30), although this was not statistically significant, possibly due to the small sample size. MACEs were substantially guided by the incidence of periprocedural myocardial infarction (5.8% vs 11.8%, p = 0.47). No patient died during the study, and unplanned target vessel re-PCI was required in one patient of the placebo arm because of acute stent thrombosis. Myocardial damage was more frequent in patients of the placebo arm than in those receiving atorvastatin reload (39.2% vs 25%, p = 0.18).
| Variable | Phase A | Phase B | ||||
|---|---|---|---|---|---|---|
| Atorvastatin (N = 27) | Placebo (N = 26) | P | Atorvastatin (N = 25) | Placebo (N = 25) | P | |
| Age (years) | 69±7 | 66±11 | 0.24 | 71±9 | 69±9 | 0.44 |
| Male | 19 (70%) | 22 (85%) | 0.36 | 17 (68%) | 23 (92%) | 0.08 |
| Hypertension | 25 (93%) | 24 (92%) | 0.63 | 25 (100%) | 20 (80%) | 0.06 |
| Diabetes mellitus | 9 (33%) | 13 (50%) | 0.34 | 13 (52%) | 14 (56%) | 1 |
| Hypercholesterolemia | 27 (100%) | 25 (96%) | 0.98 | 19 (76%) | 18 (72%) | 1 |
| Current smoker | 4 (15%) | 6 (23%) | 0.67 | 6 (24%) | 4 (16%) | 0.72 |
| Body mass index (kg/m2) | 29±3 | 28±3 | 0.23 | 27±3 | 27±3 | 1 |
| Clinical presentation | ||||||
| Stable angina pectoris | 16 (59%) | 16 (62%) | 0.91 | 20 (80%) | 22 (88%) | 0.70 |
| Acute coronary Syndrome | 11 (41%) | 10 (39%) | 0.91 | 5 (20%) | 3 (12%) | 0.70 |
| Previous myocardial infarction | 5 (18%) | 11 (42%) | 0.11 | 8 (32%) | 10 (40%) | 0.77 |
| Previous coronary intervention | 8 (30%) | 11 (42%) | 0.50 | 15 (60%) | 14 (56%) | 1 |
| Previous coronary bypass surgery | 1 (4%) | 3 (11%) | 0.58 | 3 (12%) | 2 (8%) | 1 |
| Serum creatinine (mg/dl) | 1.05±0.22 | 1.19±0.29 | 0.053 | 0.95±0.33 | 1.07±0.27 | 0.17 |
| Estimated glomerular filtration rate (ml/min/1.73m 2 ) ∗ | 75.6±22.4 | 68.9±17.9 | 0.24 | 82.4±27.3 | 75.5±27.3 | 0.38 |
| Left ventricular ejection fraction (%) | 54±9 | 55±8 | 0.67 | 57±6 | 57±6 | 1 |
| Therapy | ||||||
| Aspirin | 27 (100%) | 26 (100%) | – | 25 (100%) | 25 (100%) | – |
| Clopidogrel | 27 (100%) | 26 (100%) | – | 25 (100%) | 25 (100%) | – |
| β-blockers | 11 (41%) | 8 (31%) | 0.64 | 12 (48%) | 13 (52%) | 1 |
| Angiotensin converting enzyme inhibitors/angiotensin receptor antagonists | 20 (74%) | 19 (73%) | 0.82 | 20 (80%) | 16 (64%) | 0.34 |
| Type of statin | ||||||
| Atorvastatin | 18 (67%) | 13 (50%) | 0.34 | 19 (76%) | 18 (72%) | 1 |
| Simvastatin (+/-Ezetimibe) | 5 (15%) | 11 (42%) | 0.11 | 2 (8%) | 1 (4%) | 1 |
| Rosuvastatin | 2 (7%) | 1 (4%) | 0.97 | 4 (16%) | 6 (24%) | 0.72 |
| Pravastatin | 2 (7%) | 1 | 0.97 | 0 | 0 | – |
∗ Estimated glomerular filtration rate was calculated by Cockcroft–Gault formula.
| Variable | Phase A | Phase B | ||||
|---|---|---|---|---|---|---|
| Atorvastatin (N = 27) | Placebo (N = 26) | P | Atorvastatin (N = 25) | Placebo (N = 25) | P | |
| Multivessel coronary disease | 13 (48%) | 7 (27%) | 0.19 | 19 (76%) | 14 (56%) | 0.23 |
| Coronary vessel treated | ||||||
| Left main | 0 | 0 | – | 0 | 1 (3%) | 1 |
| Left anterior descending | 11 (33%) | 15 (50%) | 0.34 | 12 (40%) | 7 (21%) | 0.24 |
| Left circumflex | 12 (37%) | 8 (27%) | 0.46 | 6 (20%) | 16 (49%) | 0.01 |
| Right | 10 (30%) | 7 (23%) | 0.62 | 11 (37%) | 9 (27%) | 0.77 |
| Saphenous vein grafts | 0 | 0 | – | 1 (3%) | 0 | 1 |
| Lesion B2/C | 14 (51%) | 10 (38%) | 0.48 | 14 (56%) | 15 (60%) | 1 |
| Multivessel intervention | 6 (22%) | 4 (15%) | 0.78 | 5 (20%) | 8 (32%) | 0.52 |
| Type of procedure | ||||||
| Balloon only | 4 (15%) | 1 (4%) | 0.37 | 2 (8%) | 2 (8%) | 0.60 |
| Stent | 23 (85%) | 25 (96%) | 0.37 | 23 (92%) | 23 (92%) | 0.60 |
| Use of drug-eluting stents | 3 (11%) | 3 (11%) | 0.70 | 4 (16%) | 7 (28%) | 0.49 |
| Periprocedural antithrombotic therapy | ||||||
| Unfractionated heparin | 22 (81%) | 21 (81%) | 0.78 | 23 (92%) | 22 (88%) | 1 |
| Bivalirudin | 5 (19%) | 5 (19%) | 0.78 | 2 (8%) | 3 (12%) | 1 |
| Glycoprotein IIb/IIIa inhibitors | 2 (7%) | 2 (8%) | 0.63 | 4 (16%) | 2 (8%) | 0.66 |
| Total mean contrast volume (ml) | 201±82 | 206±79 | 0.82 | 146±51 | 167±71 | 0.24 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


