Percutaneous mitral valve repair (PMVR) using the MitraClip System is feasible and entails clinical improvement even in patients with high surgical risk and severe functional mitral regurgitation (MR). The aim of this study was to assess survival rates and clinical outcome of patients with severe, functional MR treated with optimal medical therapy (OMT) compared with those who received MitraClip device. Sixty patients treated with OMT were compared with a propensity-matched cohort of 60 patients who underwent PMVR. Baseline demographics and echocardiographic variables were similar between the 2 groups. The mean age of patients was 75 years, and 67% were men. The median logistic EuroSCORE and EuroSCORE II were 17% and 6%, respectively, because of the presence of several co-morbidities. The mechanism of MR was functional in all cases with an ischemic etiology in 52% of patients. Median left ventricle ejection fraction was 34%. All the patients were symptomatic for dyspnea with 63% and 12% in the New York Heart Association class III and IV, respectively. In PMVR group, the procedure was associated with safety and very low incidence of procedural complications with no occurrence of procedural and inhospital mortality. After a median follow-up of 515 days (248 to 828 days), patients treated with PMVR demonstrated overall survival, survival freedom from cardiac death and survival free of readmission due to cardiac disease curves higher than patients treated conservatively (log-rank test p = 0.007, p = 0.002, and p = 0.04, respectively). In conclusion, PMVR offers a valid option for selected patients with high surgical risk and severe, functional MR and entails better survival outcomes compared with OMT.
The appropriateness of mitral valve surgery for patients with secondary mitral regurgitation (MR) and advanced heart failure is more questionable than that for primary MR. In particular, in symptomatic patients with severe secondary MR and severely depressed systolic left ventricular (LV) function, who cannot be revascularized or who present with cardiomyopathy, the decision to operate remains ambiguous. However, when surgical risk is prohibitive, percutaneous mitral valve repair (PMVR) using the MitraClip System can be considered a new therapeutic option for patients with secondary MR who remain symptomatic despite optimal medical therapy (OMT). Recently, it has been proved that in these high-risk patients with functional MR, the MitraClip procedure is safe with low rates of hospital mortality and adverse events and with sustained improvement of hemodynamic and functional status, but its impact on survival has not been established. Up to now, there are few studies that globally report better survival outcome of percutaneous mitral valve treatment compared with OMT in patients with LV dysfunction and secondary MR. However, these studies are nonrandomized and include a wide spectrum of high-risk patients with both primary and secondary MR. In the present study, our aim was to compare outcomes of patients with high surgical risk and symptomatic functional MR treated conservatively and those treated with PMVR, using propensity matching.
Methods
From December 2009 to February 2015, 160 consecutive patients affected by symptomatic severe functional MR referred to our center for possible PMVR because of estimated high or prohibitive surgical risk were included in a prospective registry ( Figure 1 ).
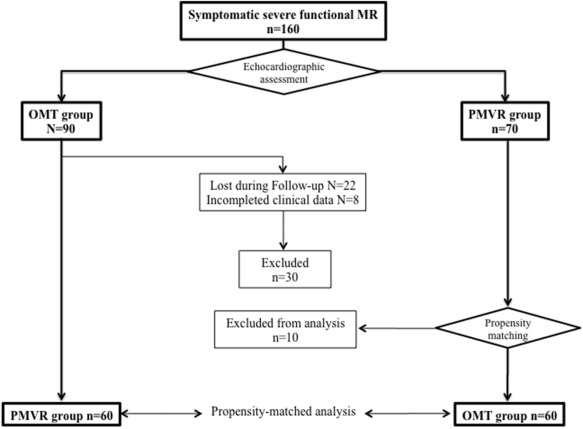
According to current guidelines, all patients were evaluated by a multispecialist team composed by cardiac surgeons, interventional and clinical cardiologists, and cardiac anesthesiologists. High surgical risk was estimated by means of the logistic EuroSCORE or by the presence of relevant risk factors associated with excessive morbidity and mortality as judged by the heart team. All patients were on stable optimized individual target heart failure medication and were treated with percutaneous angioplasty and stent implantation, implantable cardioverter defibrillator, and cardiac resynchronization devices before MitraClip screening, if indicated. We used transthoracic and transesophageal echocardiography to confirm MR severity and to assess the suitability for MitraClip repair according to the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) anatomic eligibility criteria. Seventy patients who presented favorable leaflet anatomy underwent PMVR procedure (PMVR group), whereas 90 patients who were unsuitable for PMVR were treated medically (OMT group), with OMT, according to the current guidelines. The date of screening for patients treated with standard of care was considered time 0.
Of the 90 patients included in the OMT group, 30 were excluded because of a lack of completed clinical informations or inability to contact the patients. To identify 2 comparable groups of patients, 60 remaining patients treated with OMT were selected for the analysis matching by propensity score with the PMVR group. Follow-up data were prospectively collected through doctor’s office visits or telephone contact. The study protocol was approved by the ethical committee of our institution, and all patients gave informed consent.
All enrolled patients underwent transthoracic and transesophageal echocardiography (Philips iE33; Philips Ultrasound, Andover, Massachusetts) to assess the suitability for MitraClip repair and to confirm MR severity. All measures were performed according to American Society of Echocardiography recommendations guidelines. LV ejection fraction was calculated from apical 2- and 4-chamber views using the modified Simpson’s rule. LV internal dimensions, end-diastolic diameter, and end-systolic diameter were measured at the level of the LV minor axis, approximately at the mitral valve leaflet tips, in parasternal view, using 2-dimensional–targeted M-mode echocardiography. Left atrial chamber was evaluated using left atrial area, measured from a single-plane apical imaging window. MR severity was visually estimated by color Doppler and based on regurgitant jet extension and graded by measuring vena contracta width and the proximal isovelocity surface area method, according the following score: 1+ (mild), 2+ (mild to moderate), 3+ (moderate to severe), or 4+ (severe). The right ventricular longitudinal function was obtained, in apical 4-chamber view, placing an M-mode cursor through the tricuspid annulus and measuring tricuspid annular plane systolic excursion. Systolic pulmonary artery pressure was determined from peak tricuspid regurgitation jet velocity, using the simplified Bernoulli equation and combining this value with an estimate of the right atrial pressure.
In all cases, the MitraClip procedure was performed with the patient under general anesthesia using transesophageal echocardiography and fluoroscopic guidance in the cardiac catheterization laboratory, as previously described. In brief, MitraClip system was introduced into the left atrium through a transseptal puncture and then steered until it was aligned over the origin of the regurgitant jet and advanced into the LV. The mitral leaflets were grasped, and the device was closed to approximate the leaflets. Once the resulting MR reduction was deemed satisfactory, the clip was deployed. A second clip was placed if the reduction of MR was inadequate. Acute procedural success was defined as placement of ≥1 clips resulting in MR reduction at least 1 grade.
Categorical variables were presented as frequencies and percentages and compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous variables with a normal distribution were presented as mean ± SD and were compared using the 2-tailed Student’s unpaired t test for comparison between groups, whereas those not following a normal distribution were presented as median (interquartile range [IQR]) and were compared with the Mann-Whitney test. The cumulative incidences of clinical events at follow-up were assessed using the Kaplan-Meier method, and the log-rank test was used for comparison between groups. A p value <0.05 was considered statistically significant.
Propensity score matching was used to assemble a cohort of patients with similar baseline characteristics. The propensity score was calculated using a logistic regression model that included the following variables: age, gender, logistic EuroSCORE and EuroSCORE II values, presence of diabetes mellitus, coronary artery disease and chronic renal failure, previous cardiac surgery, and LV ejection fraction values. Matching was performed by randomly selecting a patient treated conservatively and looking for the percutaneously treated patient with the nearest logit-transformed propensity score. Finally, a total number of 60 patients who underwent OMT have been matched to 60 patients who underwent PMVR using a greedy matching algorithm. All data were processed using the Number Cruncher Statistical System 2007 (NCSS, Kaysville, Utah).
Results
We compared 60 patients treated with OMT to a propensity-matched cohort of 60 patients who underwent PMVR. The propensity score used to identify a matched OMT cohort showed a good discriminative power (C-statistic 0.77). Baseline clinical characteristics of patients according to treatment management are detailed in Table 1 . Baseline demographics variables were similar between the 2 groups. The mean age of patients was 75 ± 8 years and 67% (n = 80) were men. The median logistic EuroSCORE and EuroSCORE II were 17% (11 to 28) and 6% (4 to 12), respectively. All patients were referred to our center for PMVR assessment because judged at high surgical risk due to the presence of multiple co-morbidities.
| Characteristics | All Patients (n = 120) | PMVR (n = 60) | OTM (n = 60) | P Value |
|---|---|---|---|---|
| Age (years) | 75 ± 8 | 74 ± 8 | 76 ± 8 | 0.39 |
| Men | 80 (67%) | 42 (70%) | 38 (63%) | 0.44 |
| Body mass index (kg/m 2 ) | 25 ± 4 | 25 ± 4 | 26 ± 3 | 0.09 |
| Logistic EuroSCORE | 17 (11-28) | 16 (11-30) | 17 (12-28) | 0.61 |
| EuroSCORE II | 6 (4-12) | 5 (3-15) | 7 (4-12) | 0.24 |
| Diabetes mellitus | 35 (29%) | 17 (28%) | 18 (30%) | 0.84 |
| Chronic kidney disease ∗ | 49 (41%) | 29 (48%) | 20 (33%) | 0.09 |
| Glomerular filtration rate (ml/min/1.73m 2 ) | 47 ± 19 | 48 ± 15 | 46 ± 22 | 0.59 |
| Previous stroke | 1 (0.8%) | 1 (1.6%) | 0 (0%) | 0.05 |
| Previous acute pulmonary aedema | 36 (30%) | 22 (37%) | 14 (23%) | 0.06 |
| Hypertension | 71 (59%) | 39 (65%) | 32 (53%) | 0.19 |
| Chronic obstructive pulmonary disease | 27 (23%) | 15 (25%) | 12 (20%) | 0.51 |
| Atrial Fibrillation | 47 (39%) | 21 (35%) | 26 (43%) | 0.35 |
| Coronary artery disease | 62 (52%) | 27 (45%) | 35 (58%) | 0.14 |
| Prior myocardial infarction | 45 (38%) | 22 (37%) | 23 (38%) | 0.85 |
| Previous coronary bypass | 30 (25%) | 14 (23%) | 16 (27%) | 0.67 |
| Previous percutaneous coronary intervention | 38 (32%) | 17 (28%) | 21 (35%) | 0.43 |
| Previous aortic valve surgery | 12 (10%) | 5 (8%) | 7 (12%) | 0.54 |
| Implanted device | ||||
| Implantable Cardioverter Defibrillator/ Cardiac Resynchronization Therapy | 42 (35%) | 24 (40%) | 18 (30%) | 0.25 |
| Implantable Cardioverter Defibrillator | 10 (8%) | 4 (7%) | 6 (10%) | 0.51 |
| Therapy | ||||
| Beta-blocker | 81(68%) | 40 (67%) | 41 (68%) | 0.40 |
| Angiotensin-Converting Enzyme -inhibitor/Angiotensin Receptor Blocker | 77 (64%) | 42 (70%) | 35 (58%) | 0.36 |
| Loop diuretics | 108 (90%) | 56 (93%) | 52 (87%) | 0.15 |
| Mineralcorticoid receptor antagonist | 62 (52%) | 35 (58%) | 27 (45%) | 0.17 |
| NYHA functional class | ||||
| Class III | 75 (63%) | 36 (60%) | 39 (66%) | 0.49 |
| Class IV | 14 (12%) | 8 (13%) | 6 (10%) | 0.59 |
The mechanism of MR was functional in all cases with an ischemic etiology in 52% patients. All the patients were symptomatic for dyspnea with 63% and 12% in the New York Heart Association class III and IV, respectively. Baseline echocardiographic parameters were similar between the 2 groups of patients and are detailed in Table 2 . The overall study population showed increased LV dimensions with severely decreased LV ejection fraction.
| Parameters | All Patients (n = 120) | PMVR (n = 60) | OTM (n = 60) | P Value |
|---|---|---|---|---|
| Left ventricular end diastolic diameter (mm) | 64 ± 9 | 64 ± 11 | 64 ± 7 | 0.87 |
| Left ventricular end systolic diameter (mm) | 49 ± 12 | 50 ± 13 | 49 ± 10 | 0.77 |
| Left ventricular end diastolic volume (ml) | 182 ± 63 | 187 ± 70 | 178 ± 54 | 0.42 |
| Left ventricular end systolic volume (ml) | 113 (73-149) | 115 (62-145) | 107 (84-153) | 0.77 |
| Left ventricular ejection fraction (%) | 34 (26-43) | 33 (26-49) | 34 (27-41) | 0.82 |
| Left atrial diameter (mm) | 52 (46-57) | 51 (46-55) | 52 (49-57) | 0.15 |
| Left atrial area (cm 2 ) | 30 (25-35) | 30 (25-36) | 29 (25-35) | 0.44 |
| Mitral regurgitation grade | ||||
| 3+ | 57 (49%) | 27 (45%) | 30 (53%) | 0.41 |
| 4+ | 54 (46%) | 33 (55%) | 21 (37%) | 0.05 |
| Systolic pulmonary artery pressure (mmHg) | 50 ± 11 | 51 ± 10 | 49 ± 12 | 0.47 |
| Systolic pulmonary artery pressure ≥60 mmHg | 25 (23%) | 14 (26%) | 11 (21%) | 0.60 |
| Tricuspid annulus plane systolic excursion (mm) | 18 (15-20) | 18 (14-20) | 18 (15-21) | 0.95 |
Procedural results and inhospital adverse events are defined in Table 3 . Acute procedural success, defined as placement of ≥1 clips resulting in MR reduction at least 1 grade, was obtained in 98% of patients, and 44 (92%) were discharged with an MR of grade ≤2+. Significant initial MR reduction because of MitraClip implantation was persistent during the follow-up period. At 12-month follow-up, 31 patients (72%) maintained the MR grade achieved by the intervention, 5 (12%) improved (from MR 2+ to MR 1+) and 7 (16%) worsened (from MR 2+ to MR 3+).
| Data | PMVR (n = 60) |
|---|---|
| Acute procedural sucess | 98 % |
| Procedural time (min) | 142 ± 40 |
| Device time (min) | 81 ± 42 |
| Implant ≥2 clips | 19 (32%) |
| Sepsis | 2 (3.3%) |
| Acute renal failure | 1 (1.7%) |
| New onset of atrial fibrillation | 2 (3.3%) |
| Bleeding requiring transfusion | 4 (6.6%) |
| Partial clip detachment before discharge | 3 (5%) |
| Pericardial tamponade | 0 |
| Urgent cardiovascular surgery for adverse event | 0 |
| Vascular complication requiring intervention | 0 |
| Stroke | 0 |
| Mechanical ventilation >48h | 0 |
| Myocardial infarction | 0 |
| Length of hospital stay-post procedure (days) | 5 (4-6) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


