American College of Cardiology Foundation/American Heart Association guidelines no longer recommend the use of routine aspiration thrombectomy during primary percutaneous coronary intervention (PCI). This is based on evidence from recent randomized controlled trials (RCTs) that suggests that the long-term benefits of aspiration thrombectomy were previously overestimated. We conducted a systematic review and meta-analysis of RCTs to examine the effect of routine aspiration thrombectomy during primary PCI versus primary PCI alone on markers of reperfusion immediately after PCI and on clinical outcomes at ≥6 months. We systematically searched Medline, EMBASE, and the Cochrane Library of Clinical trials for RCTs published in English or French with follow-up ≥6 months. Data were pooled using random-effects models. Eighteen publications (containing data from 14 RCTs, n = 20,285) met our inclusion criteria. Aspiration thrombectomy was associated with higher rates of ST-segment resolution (relative risk [RR] 1.22, 95% CI 1.07 to 1.40) and myocardial blush grade 3 (RR 1.30, 95% CI 1.01 to 1.67) and a reduced risk of no reflow immediately after PCI (RR 0.63, 95% CI 0.40 to 0.98). However, thrombectomy was not associated with our primary outcome of all-cause mortality at longest available follow-up (RR 0.92, 95% CI 0.81 to 1.04). Similar results were obtained for myocardial infarction and target vessel/lesion revascularization. Thrombectomy also increased the risk of stroke (RR 1.59, 95% CI 1.07 to 2.35). In conclusion, routine aspiration thrombectomy during primary PCI has some short-term clinical benefits but does not improve outcomes ≥6 months and increases the risk of stroke.
Aspiration thrombectomy during primary percutaneous coronary intervention (PCI) is associated with reduced distal embolization and improved microvascular reperfusion and was thought to improve clinical outcomes such as mortality and reinfarction. The 2013 American College of Cardiology Foundation/American Heart Association guidelines for the treatment of ST-segment elevation myocardial infarction (STEMI) recommended the use of routine aspiration thrombectomy during primary PCI. However, previous randomized controlled trials (RCTs), including those that informed the 2013 guidelines, were underpowered to detect differences in important clinical end points, such as mortality. Moreover, findings from recent RCTs suggest that previous studies may have overestimated the long-term benefits of routine aspiration thrombectomy during primary PCI. It is based on these RCTs that the American College of Cardiology Foundation/American Heart Association recently revised their guidelines and retracted their previous recommendation. Given the recent publication of long-term data, we have conducted a systematic review and meta-analysis of RCTs to synthesize the totality of the current evidence regarding the impact of aspiration thrombectomy during primary PCI versus primary PCI alone on markers of reperfusion immediately after PCI and on clinical outcomes at ≥6 months.
Methods
We conducted and reported our meta-analysis according to a prespecified protocol and report it as described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
We systematically searched MEDLINE (through Ovid), EMBASE (through Ovid), and the Cochrane Library of Clinical Trials in April 2015 for English or French language publications to identify RCTs examining aspiration thrombectomy ( Appendices 1 to 3 ). Briefly, search terms included the keywords “thrombect*,” “aspirat*,” “percutaneous coronary intervention,” “PCI,” and “angioplasty.” The searches also included Medical Subject Headings and Emtree terms such as “Thrombectomy,” “Percutaneous Coronary Intervention,” and “Myocardial Infarction” where applicable. A modified version of the McMaster RCT hedge was used to limit our MEDLINE and EMBASE searches to RCTs. We did not limit our search by date of publication. We additionally hand-searched the references of relevant RCTs, reviews, and meta-analyses retrieved by our electronic database searches for potentially eligible RCTs.
The title and abstract of identified publications were screened according to prespecified inclusion and exclusion criteria, with all potentially relevant studies retrieved for full-text review. We included RCTs with follow-up ≥6 months and that randomized patients with STEMI to adjunctive aspiration thrombectomy during primary PCI or to primary PCI alone. Primary PCI was defined as PCI conducted emergently as the first form of treatment for STEMI, without the administration of fibrinolytic therapy. RCTs in which a similar portion of participants in each study arm underwent rescue PCI (i.e., PCI after administration of fibrinolytics) were eligible for inclusion, whereas RCTs exclusively investigating PCI after fibrinolytic therapy were not. RCTs examining both aspiration and mechanical thrombectomy during primary PCI were included provided that the data were stratified according to the device used. We excluded RCTs investigating thrombectomy during PCI of saphenous vein grafts.
Two independent reviewers extracted data from eligible RCTs using a standardized and pilot-tested data collection form. Disagreements were resolved by consensus or, when necessary, by a third reviewer. Extracted data included the thrombectomy device used; the preprocedural and/or periprocedural administration of aspirin, antiplatelet therapies, and glycoprotein IIb/IIIa inhibitors (GPIs); the mean age of participants; and the percentage of men per treatment arm. All outcome data were extracted at maximum follow-up, as well as at 1, 6, and 12 months after PCI, if available. The primary end point was all-cause mortality at the longest follow-up reported. Secondary end points included all-cause mortality at other follow-ups, cardiovascular-related mortality, major adverse cardiovascular events (MACE), stroke, postprocedural Thrombolysis In Myocardial Infarction (TIMI) 3 flow, myocardial blush grade 3, no reflow, and ST-segment resolution within 90 minutes of PCI.
The quality of included RCTs was assessed using the Cochrane Collaboration’s tool for assessing risk of bias in RCTs. Quality assessment was performed by 2 independent reviewers, with disagreements resolved by consensus. Eligible RCTs were included in the meta-analysis regardless of their assessed quality.
We used DerSimonian and Laird random-effects models to estimate relative risks (RRs) and 95% CIs. Random-effects analyses were also used to calculate risk differences (RDs). Analyses were intention-to-treat for all outcomes, and a continuity correction was used to include zero-event trials. Trials with factorial designs were analytically treated as 2 separate trials. In sensitivity analyses, fixed-effects models were used. In addition, influence analyses examined the effect of individual studies on the overall meta-analytic estimates. We assessed the potential for publication bias using a funnel plot and Egger’s test for small effects. All analyses were conducted using StataSE 14.1 (StataCorp LP, College Station, Texas).
Results
Our literature search yielded 897 publications ( Figure 1 ). After the removal of duplicates, the titles and abstracts of 553 publications were screened. Of these, 49 full-text studies were retrieved and screened for eligibility, with 17 studies (containing data from 14 RCTs) meeting inclusion criteria (n = 20,285). The 12-month data of one of these RCTs were recently published and have also been included, bringing the number of studies meeting inclusion criteria up to 18.

Most included RCTs (n = 10) were conducted in North America or Europe, whereas 3 were conducted in Asia and 1 in Egypt ( Table 1 ). Maximum follow-up ranged from 6 to 24 months, with 1-, 6-, and 12-month data reported in 4, 6, and 5 trials, respectively. The most commonly used aspiration device was the Export aspiration catheter (Medtronic, Minneapolis, Minnesota), which was used in 9 RCTs. Other devices included the Diver CE (Invatec, Italy), Thrombuster II (Kaneka, Japan), and TransVascular Aspiration Catheter (Nipro, Japan). The inclusion criteria of all included studies required that participants exhibit ST-segment elevation in 2 contiguous leads on an electrocardiogram at presentation. Across all trials, there was variation in the inclusion criterion of maximum duration of ischemic symptoms (median: 12 hours; range: 5 to 24 hours).
| Trial ∗ | Device | n | Mean Age, yrs | Male | DM | HTN | GPI Use | Duration, months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tx + PCI | PCI alone | Tx + PCI | PCI alone | Tx + PCI | PCI alone | Tx + PCI | PCI alone | Tx + PCI | PCI alone | Tx + PCI | PCI alone | |||
| TOTAL | Export | 5,033 | 5,030 | 61.0 | 61.0 | 76.8% | 76.8% | 18.3% | 18.6% | 50.3% | 50.0% | 37.4% | 41.4% | 6 |
| TASTE | Export | 3,621 | 3,623 | 65.9 | 66.5 | 75.1% | 74.6% | 12.4% | 12.5% | NR | NR | 15.4% | 17.4% | 12 |
| TAPAS | Export | 535 | 536 | 63.0 | 63.0 | 67.9% | 73.1% | 10.6% | 12.6% | 33.1% | 37.1% | 93.4% | 89.9% | 12 |
| INFUSE-AMI | Export | 229 | 223 | 61.0 † | 59.0 † | 73.8% | 74.0% | 14.5% | 7.6% | 33.2% | 29.6% | 50.0% | 50.0% | 12 |
| VAMPIRE | TVAC | 180 | 175 | 63.2 | 63.5 | 80.6% | 77.7% | 23.3% | 29.9% | 54.8% | 59.0% | 0.0% | 0.0% | 8 |
| MUSTELA ‡ | Export | 104 | 104 | 62.4 † | 63.0 † | 88.4% | 76.0% | 19.2% | 20.4% | 51.9% | 47.6% | 100% | 100% | 12 |
| PIHRATE | Diver CE | 100 | 96 | 60.8 | 58.8 | 80.0% | 76.8% | 13.0% | 9.6% | 58.0% | 53.7% | 8.0% | 10.5% | 6 |
| EXPIRA | Export | 88 | 87 | 66.7 | 64.6 | 64.7% | 55.1% | 23.8% | 18.6% | 67.0% | 49.4% | 100% | 100% | 24 |
| Liistro et al. | Export | 55 | 56 | 64.0 | 65.0 | 78.1% | 76.8% | 20.0% | 12.5% | 60.0% | 53.6% | 100% | 100% | 6 |
| ITTI | Thrombuster | 52 | 48 | 59.6 | 57.6 | 90.4% | 81.3% | 26.9% | 25.0% | 51.9% | 60.4% | 53.8% | 52.1% | 6 |
| Shehata | Export | 50 | 50 | 60.3 | 59.4 | 62.0% | 66.0% | 100% | 100% | 60.0% | 64.0% | 100% | 100% | 8 |
| De Luca et al. | Diver CE | 38 | 38 | 66.7 | 64.6 | 71.1% | 55.3% | 23.7% | 18.4% | 39.5% | 50.0% | 100% | 100% | 6 |
| Chao et al. | Export | 37 | 37 | 60.0 | 63.0 | 83.8% | 86.5% | 32.4% | 21.6% | 56.8% | 56.8% | 18.9% | 32.4% | 6 |
| Bulum et al. | Export | 30 | 30 | 54.3 | 58.2 | 83.3% | 73.3% | 10.0% | 10.0% | 40.0% | 43.3% | 96.7% | 83.3% | 6 |
∗ Trials are presented in order of descending size of study population.
‡ The data from participants randomized to aspiration thrombectomy were obtained from a previously published meta-analysis.
Approximately 3 quarters of participants were men, with the mean age ranging from 54.3 to 66.7 years. The vast majority of RCTs administered dual antiplatelet therapy to 98% to 100% of participants. The use of GPIs varied across trials and was generally used at the discretion of the operator. In the INFUSE-Anterior Myocardial Infarction (INFUSE-AMI) and Initial Thrombosuction and Tirofiban Infusion (ITTI) trials, GPI use was part of their factorial designs, in which patients were randomized to either thrombectomy or primary PCI alone and to the use or nonuse of a GPI.
Overall, the included RCTs were assessed to have a low or unclear risk of bias in most domains ( Appendix 4 ). One RCT was found to have a high risk of bias due to incomplete outcome reporting, whereas another had a high risk of bias in the domain of blinding.
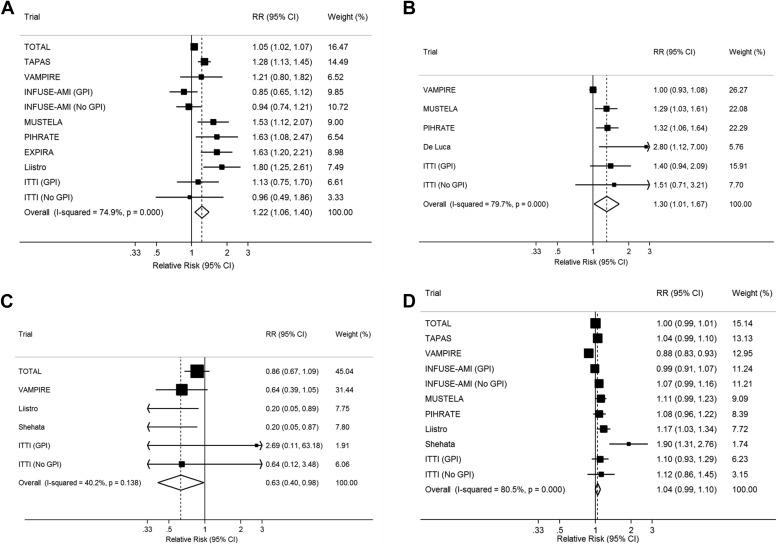
Aspiration thrombectomy during primary PCI resulted in significant improvements in certain surrogate markers of myocardial reperfusion immediately after PCI, compared with primary PCI alone. These benefits included a significantly higher risk of ST-segment resolution within 90 minutes of PCI (RR 1.22, 95% CI 1.07 to 1.40; Figure 2 ), an increased risk of myocardial blush grade 3 (RR 1.30, 95% CI 1.01 to 1.67; Figure 2 ), and a reduced risk of no reflow after PCI (RR 0.63, 95% CI 0.40 to 0.98; Figure 2 ). No significant differences were observed in postprocedure TIMI flow ≥3 (RR 1.04, 95% CI 0.99 to 1.10; Figure 2 ).
In contrast, aspiration thrombectomy did not show any significant improvement in most clinical outcomes within 1 month of primary PCI, including all-cause mortality (RR 0.89, 95% CI 0.69 to 1.13; Appendix 5 , Table 2 ), target vessel revascularization (RR 0.80, 95% CI 0.61 to 1.05; Appendix 6 ), stent thrombosis (RR 0.65, 95% CI 0.24 to 1.74; Appendix 7 ), and MACE (RR 0.77, 95% CI 0.54 to 1.09; Appendix 8 ). However, aspiration thrombectomy was associated with a reduced risk of MI (RR 0.57, 95% CI 0.35 to 0.93; Appendix 9 ). In addition, we observed an increased risk of stroke at 1 month (RR 2.31, 95% CI 1.92 to 2.78; Appendix 10 ) although this analysis was based on data from only 2 trials, Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI (TOTAL) and INFUSE-AMI.
| Outcome | 1 month | 6 months | 12 months | Longest Follow-up | |||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | RD (95% CI) | RR (95% CI) | RD (95% CI) | RR (95% CI) | RD (95% CI) | RR (95% CI) | |
| All-Cause Mortality | 0.89 (0.69, 1.13) | -0.004 (-0.011, 0.003) | 0.96 (0.80, 1.15) | -0.002 (-0.009, 0.006) | 0.89 (0.75, 1.06) | -0.006 (-0.016, 0.004) | 0.92 (0.81, 1.04) |
| CV Mortality | NA | NA | 0.94 (0.77, 1.14) | -0.002 (-0.009, 0.005) | 0.74 (0.43, 1.28) | -0.014 (-0.042, 0.014) | 0.72 (0.44, 1.16) |
| MI | 0.57 (0.35, 0.93) | -0.004 (-0.008, 0.000) | 1.03 (0.81, 1.31) | 0.001 (-0.005, 0.007) | 0.97 (0.79, 1.18) | -0.001 (-0.006, 0.004) | 0.95 (0.80, 1.13) |
| Stroke | 2.31 (1.92, 2.78) | 0.011 (-0.026, 0.049) | 1.66 (1.11, 2.48) | 0.005 (0.001, 0.009) | 1.59 (1.07, 2.38) | 0.004 (0.001, 0.008) | 1.59 (1.07, 2.35) |
| TVR | 0.80 (0.61, 1.05) | -0.005 (-0.011, 0.001) | 1.06 (0.90, 1.25) | 0.003 (-0.005, 0.012) | 0.97 (0.84, 1.12) | -0.002 (-0.011, 0.006) | 0.98 (0.87, 1.10) |
| TLR | NA | NA | 0.65 (0.34, 1.25) | -0.014 (-0.040, 0.012) | NA | NA | 0.83 (0.67, 1.02) |
| Stent Thrombosis | 0.65 (0.24, 1.74) | -0.003 (-0.005, 0.000) | 0.88 (0.63, 1.22) | -0.002 (-0.006, 0.003) | 0.84 (0.64, 1.09) | -0.002 (-0.006, 0.002) | 0.83 (0.64, 1.07) |
| MACE | 0.77 (0.54, 1.09) | -0.020 (-0.046, 0.007) | 0.97 (0.86, 1.11) | -0.001 (-0.011, 0.009) | 0.94 (0.86, 1.03) | -0.004 (-0.013, 0.005) | 0.91 (0.84, 0.99) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


