The purpose of the study was to evaluate the optimal duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention, especially in the era of second-generation drug-eluting stents (DES). The work was conducted from November 2014 to April 2015. All randomized controlled trials comparing short (<12 months) versus long (≥12 months) DAPT in patients treated with second-generation DES were analyzed. Sensitivity analyses were performed for length of DAPT and type of DES. All-cause death was the primary end point, whereas cardiovascular death, myocardial infarction (MI), stent thrombosis (ST), and major bleeding were secondary end points. Results were pooled and compared with random-effect models and meta-regression analysis. Eight randomized controlled trials with 18,810 randomized patients were included. The studies compared 3 versus 12 months of DAPT (2 trials), 6 versus 12 months (3 trials), 6 versus 24 months (1 trial), 12 versus 24 months (1 trial), and 12 versus 30 months (1 trial). Comparing short versus long DAPT, there were no significant differences in all-cause death (odds ratio [OR] 0.87; 95% confidence interval [CI] 0.66 to 1.44), cardiovascular death (OR 0.95; 95% CI 0.65 to 1.37), and ST (OR 1.20; 95% CI 0.79 to 1.83), and no differences were present when considering everolimus-eluting and fast-release zotarolimus-eluting stents separately. Shorter DAPT was inferior to longer DAPT in preventing MI (OR 1.35; 95% CI 1.03 to 1.77). Conversely, major bleeding was reduced by shorter DAPT (OR 0.60; 95% CI 0.42 to 0.96). Baseline features did not influence these results in meta-regression analysis. In conclusion, DAPT for ≤6 months is reasonable for patients treated with everolimus-eluting and fast-release zotarolimus-eluting stents, with the benefit of less major bleeding at the cost of increased MI, with similar survival and ST rates. An individualized patient approach to DAPT duration should take into account the competing risks of bleeding and ischemic complications after present-generation DES.
Selecting the optimal duration of dual antiplatelet therapy (DAPT) is a challenge for physicians managing patients treated with drug-eluting stents (DES). As the indications for percutaneous coronary intervention (PCI) have expanded, patients are being treated with greater co-morbidities and/or complex coronary anatomies. Such patients may theoretically benefit from longer DAPT to prevent ischemic complications including myocardial infarction (MI) and stent thrombosis (ST), although bleeding may be increased. In this regard, ST rates have been reduced with current second-generation DES compared with first-generation platforms, which may change the risk-benefit equation for prolonged DAPT. In addition, first-generation sirolimus-eluting stents and paclitaxel-eluting stents are either no longer manufactured or rarely used, respectively, and DAPT duration data with these devices are, thus, no longer relevant for current practice. Current evidences suggest a benefit in reduction of MI (both in native vessels and in-stent) for longer DAPT with contrasting data about mortality but are fraught by including together first- and second-generation stents and not performing a sensitivity analysis for different types of stent. We, therefore, performed an updated meta-analysis to appraise the safety and efficacy of different durations of DAPT in patients treated with second-generation DES.
Methods
The present study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements (see Supplementary Material for more details). From November 2014 to April 2015, Pubmed, Cochrane, and Google Scholar were searched for the following terms: “dual antiplatelet therapy” and “coronary” and “stent” and “second generation” by 2 authors (FDA and CM). Citations were first screened independently by 2 reviewers (GBZ and FDA), with disagreements resolved by consensus. Inclusion criteria were (i) human studies, (ii) investigating patients who underwent coronary revascularization with PCI and second-generation DES, (iii) comparing different length of DAPT: <12 months (“short”) and ≥12 months (“long”), and (iv) with separate data reported for second-generation DES (either in the manuscript or available from the investigators). In the case of duplicate reporting, the manuscript with the largest sample of patients was selected.
The following data were independently abstracted by 2 reviewers (GBZ and FDA) on prespecified electronic forms, with disagreements resolved by consensus: authors, journal, year of publication, location of the study group, type of DES, baseline, angiographic and procedural features, length of DAPT, and definition of bleeding were collected. The corresponding authors of the relevant studies were queried for required quantitative details not in the published manuscripts.
The primary end point was all-cause death, whereas secondary end points included cardiovascular death, MI, definite or probable ST, target vessel revascularization (TVR), and major bleeding. Sensitivity analyses were performed for stent type (everolimus-eluting stents [EES] and fast-release zotarolimus-eluting stents [ZES]) and for DAPT duration (≤6 vs 12 months; ≤6 vs 24 months; 12 vs ≥24 months).
The quality of included studies was independently appraised by 2 reviewers (GBZ and FDA), with disagreements resolved by consensus. For each randomized controlled trial, we evaluated the risk of bias (low, unclear, or high) for random-sequence generation, allocation concealment, blinding of patients and physicians, blinding during assessment of follow-up, incomplete outcome evaluation, and selective reporting, in keeping with the Cochrane Collaboration approach.
Continuous variables are reported as mean (SD) or median (first and third quartile). Categorical variables are expressed as n (%). Statistical pooling for incidence estimates was performed according to a random-effect model with generic inverse-variance weighting, computing risk estimates with 95% confidence intervals (CIs), using RevMan 5.2 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Small study bias was appraised by graphical inspection of funnel plots. Meta-regression analysis was performed to assess the impact of baseline features on the primary end point with Comprehensive Meta-analysis software (trial version).
Hypothesis testing for superiority was set at the 2-tailed 0.05 level. Hypothesis testing for statistical homogeneity was set at the 2-tailed 0.10 level and based on the Cochran Q test, with I 2 values of 25%, 50%, and 75% representing mild, moderate, and severe heterogeneity, respectively.
Results
As shown in Figure 1 , 1,632 publications were found at the initial search, and after abstract evaluation, 10 articles were appraised as full texts. Two of these were excluded because separate data for second-generation stents were not reported. Finally, 8 studies with 18,810 randomized patients were included in the present analysis. With regard to the prolonging dual antiplatelet treatment after grading stent-induced intimal hyperplasia (PRODIGY) trial, we limited the analysis to patients randomized to receive ZES or EES. Similarly, we included only reported data on second-generation DES from the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) and DAPT trials. For the Optimal Duration of Clopidogrel Therapy with DES to Reduce Late Coronary Arterial Thrombotic Event (DES LATE) and Second-Generation Drug-Eluting Stent Implantation Followed by 6- Versus 12-Month Dual Antiplatelet Therapy (SECURITY) trials, data specific for second-generation stents were provided by the investigators.
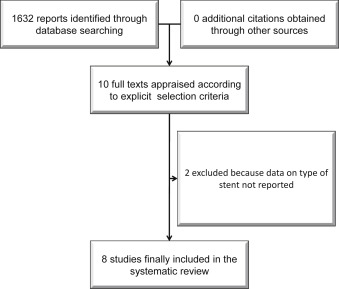
Table 1 lists the main features of the studies. Of the 18,810 patients, 12,510 were treated with EES, 5,768 were treated with fast-release ZES, and 532 were treated with biolimus-eluting stents. The 8 trials compared 3 versus 12 months DAPT (2 trials), 6 versus 12 months DAPT (3 trials), 6 versus 24 months DAPT (1 trial), 12 versus 24 months (1 trial), and 12 versus 30 months DAPT (1 trial). The majority of patients were male, with an age between 61 and 68 years ( Table 2 ). Approximately one-quarter had diabetes and approximately half presented with unstable angina or non-ST-segment elevation MI. Multivessel disease was present in approximately half of patients, and the left anterior descending was the most common target coronary artery ( Table 3 ). The baseline and angiographic features of the included patients are presented in Tables 1 and 2 .
| Trial and citation | N°patients randomized to second generation DES | Months of DAT | First Generation DES | Cypher | Endeavor/ Resolute | Promus/ Xience | Nobori | Biomatrix | Bleeding’s definition | Design of the trial | Timing of enrollement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DAPT, 14 | 5967 | 12 vs. 30 | – | 11% | 13% | 47% | – | – | BARC types 3 and 5 | Non inferiority | Within 72 hours of PCI |
| DES LATE, 14 | 2137 | 12 vs 24 | 73% | – | 11% | 16% | – | – | TIMI | – | Not detailed |
| ITALIC, 14 | 2031 | 6 vs. 24 ∗ | – | – | – | 100% Xience | – | – | REPLACE/GUSTO | Non inferiority | Not detailed |
| SECURITY, 14 EES ZES | 1372 382 809 | 6 vs. 12 | – | – | 41,2% | 20,1% | 26,3% | 12,4% | TIMI | Non inferiority | Not detailed |
| OPTIMIZE, 13 | 3119 | 3 vs. 12 | – | – | 100% Endeavor | – | – | – | – | Non inferiority | Not detailed |
| EXCELLENT, 13 | 1079 | 6 vs. 12 | – | – | – | 100% | – | – | – | Non inferiority | Not known |
| PRODIGY, ZES 13 | 493 | 6 vs. 24 | – | – | 100% Endeavor | – | – | – | BARC | Non inferiority | After 30 days |
| PRODIGY, EES 13 | 495 | 6 vs. 24 | – | – | – | 100% | – | – | BARC | Non inferiority | After 30 days |
| RESET, 12 | 2117 | 3 vs. 12 | – | . | 100% Endeavor | – | – | – | BARC types 2,3,5 and GUSTO | Superiority | After stent implantation |
| Trial and citation | Age (years) | Female | Diabetes mellitus | Ejection fraction | Acute coronary syndrome |
|---|---|---|---|---|---|
| DAPT, 14 | 62 | 25% | 31% | – | 43% |
| DES LATE, 14 | 62 | 30% | 28% | 60% | 60% |
| ITALIC, 14 | 62 | 20% | 37% | – | – |
| SECURITY, 14 | 65 | 23% | 30% | 56% | 38% |
| EES | 64 | 22% | 28% | 56% | 37% |
| ZES | 66 | 21% | 32% | 55% | 39% |
| OPTIMIZE, 13 | 61 | 36% | 35% | – | 32% |
| EXCELLENT, 13 | 63 | 35% | 38% | – | 48% |
| Prodigy, ZES 13 | 67 | 21% | 36% | 51% | 74% |
| Prodigy, EES 13 | 68 | 21% | 26% | 50% | 77% |
| RESET, 12 | 62 | 36% | 29% | 64% | 54% |
| Multivessel disease (%) | Left anterior descending target (%) | Bifurcation target (%) | Class C lesions (%) | Lesion length (mm) | |
|---|---|---|---|---|---|
| DAPT, 14 | – | 41 | 13 | 43 | – |
| ITALIC, 14 | 48 | 73 | – | – | – |
| DES LATE, 14 | 30 | 51 | 14 | – | 30 |
| SECURITY, 14 | 44 | 43 | 14 | 21 | 18 |
| OPTIMIZE, 13 | 25 | 48 | 15 | 37 | 18 |
| EXCELLENT, 13 | 51 | 52 | 10 | 53 | 23 |
| Prodigy, ZES 13 | 73 | 57 | – | 64 | 13 |
| Prodigy, EES 13 | 65 | 59 | – | 68 | 13 |
| RESET, 12 | 42 | 53 | 0 | 67 | 20 |
The major results are shown in Figures 2–7 . Comparing short versus long DAPT, there were no significant differences in all-cause death (odds ratio [OR] 0.87; 95% CI 0.66 to 1.44), cardiovascular death (OR 0.95; 95% CI 0.65 to 1.37), ST (OR 1.20; 95% CI 0.79 to 1.83), or TVR (OR 0.96; 95% CI 0.72 to 1.27), with no heterogeneity except for TVR. Shorter DAPT compared with longer DAPT was associated with a greater risk of nonfatal MI (OR 1.35; 95% CI 1.03 to 1.77). Mild heterogeneity was present, and the relation between DAPT duration and MI was mainly driven by the outcomes from the DAPT trial. The risk of MI was increased with 12 versus ≥30 months DAPT (OR 1.61; 95% CI 1.16 to 2.22), whereas there was no significant difference in ≤6 versus 12 months DAPT (OR 1.27; 95% CI 0.93 to 1.74) or for 6 versus 24 months DAPT (OR 0.81; 95% CI 0.13 to 4.87). Conversely, major bleeding was significantly less with shorter compared with longer DAPT (OR 0.60; 95% CI 0.42 to 0.96), with no heterogeneity present.
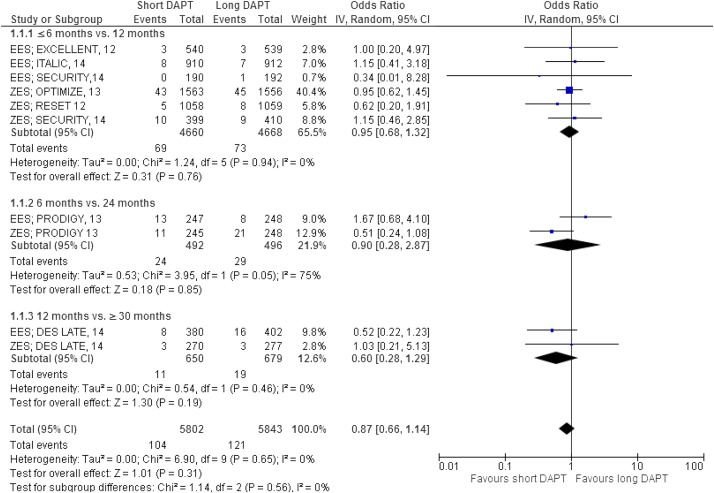
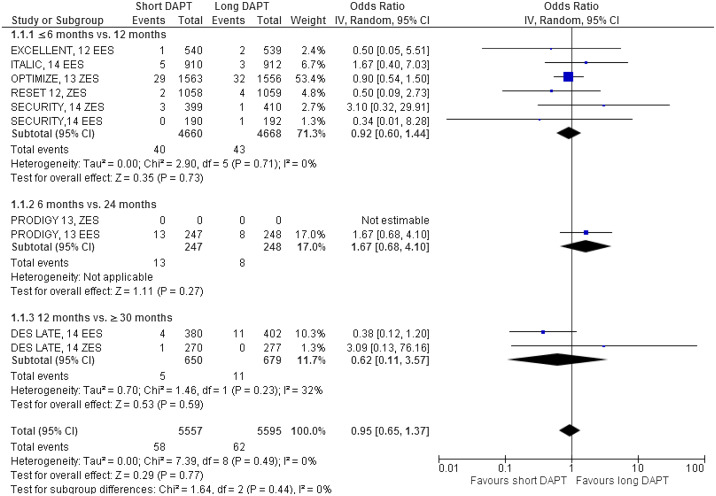
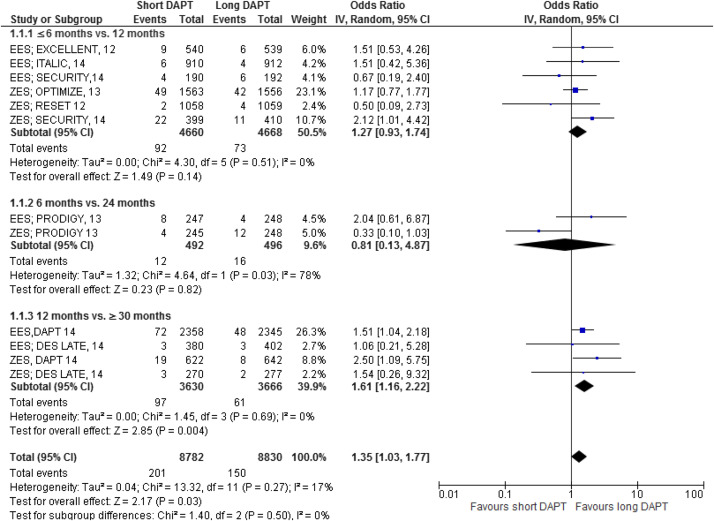
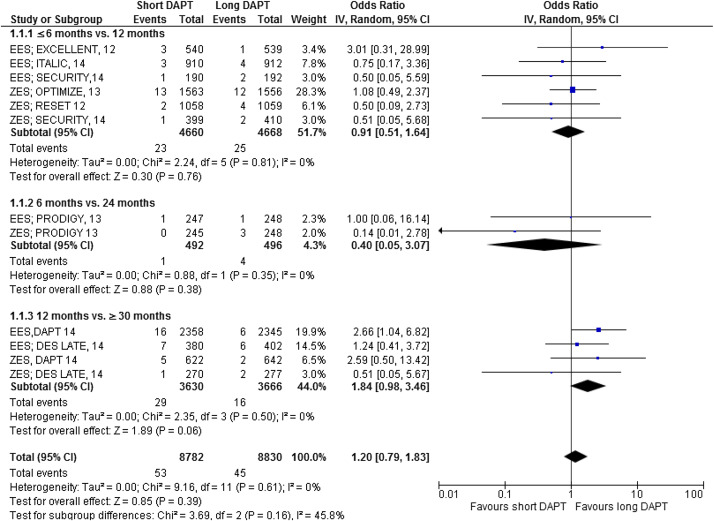
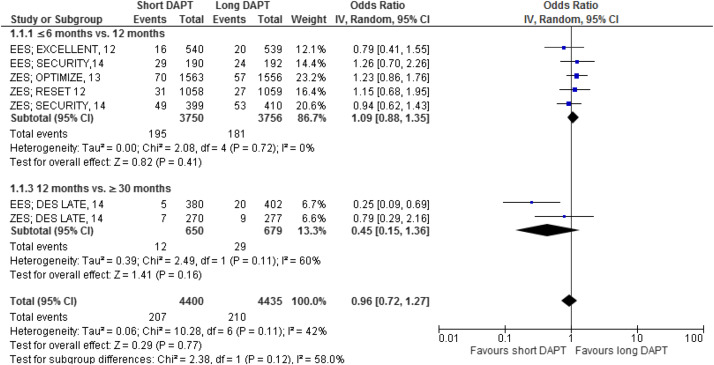
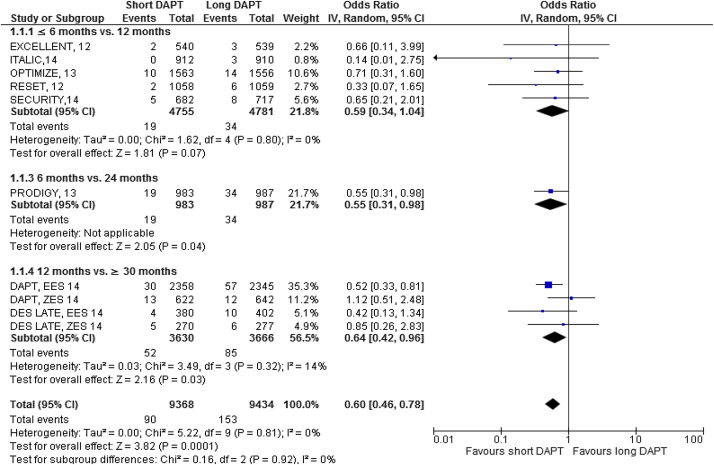
The impact of DAPT duration on ischemic and bleeding end points was consistent when considered separately for EES and fast-release ZES. By meta-regression analysis, baseline features did not influence the results for all-cause death, MI, ST, or major bleeding ( Table 4 ). There was no systematic bias apparent as assessed by funnel plot inspection and with Egger’s test that was not significant ( Figure 8 ).
| Beta | LCI (95%) | UCI (95%) | P value | |
|---|---|---|---|---|
| All-cause death | ||||
| Age ∗ | 0.01 | -0.10 | 0.13 | 0.67 |
| Female gender | -0.01 | -0.05 | 0.04 | 0.85 |
| Diabetes mellitus | -0.02 | -0.09 | 0.05 | 0.52 |
| Acute coronary syndrome | -0.02 | -0.10 | 0.14 | 0.77 |
| Multivessel disease | -0.01 | -0.08 | 0.19 | 0.65 |
| LAD | -0.03 | -0.09 | 0.04 | 0.39 |
| Class B2/C lesions | -0.08 | -0.13 | 0.15 | 0.53 |
| Lesion length † | -0.01 | -0.20 | 0.21 | 0.84 |
| Myocardial infarction | ||||
| Age ∗ | -0.06 | -0.18 | 0.45 | 0.92 |
| Female gender | -0.01 | -0.05 | 0.02 | 045 |
| Diabetes mellitus | -0.02 | -0.09 | 0.05 | 0.52 |
| Acute coronary syndrome | -0.05 | -0.03 | 0.34 | 0.67 |
| Multivessel disease | -0.01 | -0.08 | 0.21 | 0.45 |
| LAD | 0.01 | -0.05 | 0.08 | 0.98 |
| Class B2/C lesions | -0.09 | -0.17 | 0.21 | 0.24 |
| Lesion length † | 0.03 | -0.04 | 0.56 | 0.32 |
| Major bleeding | ||||
| Age ∗ | -0.04 | -0.15 | 0.06 | 0.46 |
| Female gender | 0.01 | -0.03 | 0.05 | 0.59 |
| Diabetes mellitus | 0.01 | -0.04 | 0.07 | 0.52 |
| Acute coronary syndrome | -0.02 | -0.10 | 0.01 | 0.37 |
| Multivessel disease | -0.01 | -0.03 | 0.02 | 0.29 |
| LAD | -0.03 | -0.10 | 0.05 | 0.42 |
| Class B2/C lesions | -0.08 | -0.24 | 0.21 | 0.37 |
| Lesion length † | -0.02 | -0.11 | 0.31 | 0.45 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


