Cilostazol is a generic drug with antiplatelet and antiproliferative effects. It is unclear whether adding cilostazol to standard dual antiplatelet therapy (aspirin and clopidogrel) after percutaneous coronary intervention reduces restenosis and improves the outcomes. We, therefore, conducted a systematic review and meta-analysis. We systematically searched the Cochrane Library, EMBASE, and MEDLINE databases for randomized controlled trials comparing dual antiplatelet therapy with and without cilostazol after percutaneous coronary intervention. The data were pooled using random-effects models and stratified into short-term (1-month), midterm (1- to 12-month), and long-term (≥12-month) follow-up durations. Twelve randomized controlled trials involving 5,655 patients met our inclusion criteria. The addition of cilostazol to dual antiplatelet therapy was not associated with a significant change in target lesion revascularization (TLR) and target vessel revascularization (TVR) at short-term follow-up. However, TLR and TVR were significantly reduced at midterm follow-up (relative risk 0.57, 95% confidence interval 0.39 to 0.84, and relative risk 0.62, 95% confidence interval 0.47 to 0.83, respectively). Data regarding TLR and TVR at long-term follow-up were limited and inconclusive. We did not find a difference in myocardial infarction, mortality, or major bleeding at any follow-up duration. In conclusion, the addition of cilostazol to dual antiplatelet therapy after percutaneous coronary intervention has favorable effects on TLR and TVR at 1 to 12 months, with no differences in adverse outcomes at any follow-up duration.
Randomized controlled trials (RCTs) examining the benefit of adding cilostazol to dual antiplatelet therapy (DAT; aspirin and clopidogrel) after percutaneous coronary intervention (PCI) have yielded conflicting or inconclusive results. Attempts to systematically pool these results have been limited. First, these previous systematic reviews and meta-analyses generated wide confidence intervals owing to the relatively small number of patients included. A number of larger, recently published RCTs have yet to be incorporated into a meta-analysis. Second, these meta-analyses did not pool outcomes of interest, or they pooled older studies evaluating the addition of cilostazol to a mix of treatment regimens that are less relevant for current PCI practice. We therefore conducted an updated systematic review and meta-analysis of RCTs to evaluate the effect on clinical outcomes and safety of adding cilostazol to DAT after PCI.
Methods
We systematically searched the Cochrane Library, EMBASE, and MEDLINE databases from inception through June 2011 to identify all RCTs comparing DAT with and without cilostazol after PCI. The MeSH search string for this data search was “cilostazol AND aspirin AND clopidogrel.” We searched without language restrictions and limited our search to studies conducted of humans and published in peer-reviewed journals. We manually searched reviews and references of retrieved studies for potentially relevant publications that were not identified in the database search. All retrieved studies were examined to eliminate overlapping data.
A study was included in our systematic review if it was a trial that randomly assigned patients, in a parallel-group design, to either aspirin and clopidogrel or aspirin, clopidogrel, and cilostazol, and reported the clinical outcomes. We included studies whether they were open-label, blinded, or double-blinded but assessed the risk of bias. Conference abstracts were excluded, because their results might not be final.
Two reviewers (S.F. and A.S.) independently extracted the data from each study. The results were compared, and any disagreements were resolved by consensus. The data extracted for each RCT included the first author, year of publication, country, follow-up duration, number of participants and their characteristics, and drug protocol. The pooled outcomes were target lesion revascularization (TLR), target vessel revascularization (TVR), myocardial infarction, mortality, and major bleeding. We qualitatively compared selected outcomes of interest if pooling was not appropriate.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses of RCTs. The quality of each study was evaluated using the Cochrane tool for assessing risk of bias. We assigned each a score of low, unclear, or high risk of bias to each of 6 criteria (sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias) for each trial. From these individual scores, we also assigned a summary score of low, unclear, or high risk of bias for each trial. The presence of publication bias was assessed by visual inspection of funnel plots (plots of effect estimates against sample size), which are usually skewed in the presence of publication bias, typically with overrepresentation of significant or “positive” studies.
We used DerSimonian and Laird random-effect models, which account for both within-study and between-study variability, to estimate the pooled relative risk (RR), with the 95% confidence intervals (CIs). The RR cannot be defined in this type of model if there are no events in both arms; thus, RCTs with no events in both arms were discarded from the pooled analysis of a given outcome. Forest plots were created for each outcome. Statistical heterogeneity was assessed using the Q statistic (with p <0.1 considered significant), and I 2 statistics were calculated to estimate the proportion of variance due to between-study heterogeneity. All analyses were conducted using Stata, version 9.0 (StataCorp, College Station, Texas).
Results
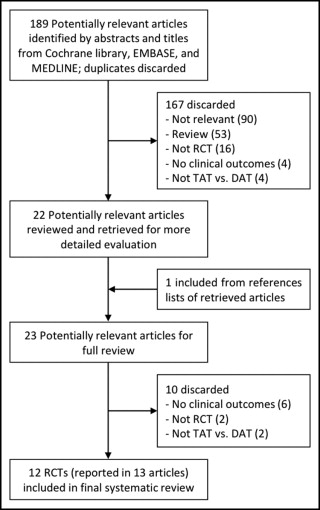
Our search of published reports identified 189 studies, excluding duplicates ( Figure 1 ) . After reviewing the titles and abstracts to exclude nonrelevant studies, case reports, design papers, and reviews, 22 studies were retrieved for additional consideration. One additional study was found from a manual search of the references of relevant reports. We then excluded observational studies, retrospective subgroup analyses, and studies that did not report the clinical outcomes of DAT with and without cilostazol. Three publications each reported outcomes at 2 follow-up periods. The long-term outcomes of 2 RCTs were reported together in a subsequent publication. Ultimately, 12 RCTs reported in 13 publications were included in our systematic review.
A summary of the included RCTs is presented in Table 1 . These trials randomized a total of 5,655 patients. Within each study, the DAT group received aspirin and clopidogrel, and the treatment group received cilostazol plus DAT. The included studies ranged in size from 60 to 1,212 patients, included predominantly male patients (58% to 75%), with a mean or median age of 58 to 67 years. The follow-up duration ranged from 1 to 24 months. Two RCTs examined only bare metal stents, 6 only drug-eluting stents, and the remaining examined both or did not specify.
| Study | Population | Age (yr) | Men (%) | Follow-Up (mo) | Drug Protocol ⁎ | ||
|---|---|---|---|---|---|---|---|
| Cilostazol | Aspirin | Clopidogrel | |||||
| Lee 2011 | DES (Z), native long lesions | 62 | 71 | 12 | LD, MD for 8 mo | LD, MD 200 mg | LD, MD |
| Suh 2011 | DES | 64 | 71 | 6 | LD, MD | LD 300 mg, MD | LD 300–600 mg, MD |
| Ahn 2010 | Stent, ACS | 64 | 62 | 24 | LD, MD | LD, MD | LD, MD |
| Lee 2010 † | DES (P or S), | 61 | 61 | 24 | LD, MD for 6 mo | LD, MD 200 mg | LD, MD for ≥6 mo |
| Han 2009 | DES (52%), BMS, ACS | 60 | 73 | 1, 12 | MD for 6 mo | MD 300 mg for 1 mo, then 100 mg | LD 300–600 mg, MD for 3–12 mo |
| Kum 2009 | DES | 62 | 75 | 1, 6 | MD for 1 mo | LD 300 mg, MD | LD, MD |
| Kim 2008 | DES | 67 | 58 | 6 | MD 50 mg twice daily | MD (+LD 300 mg for unplanned stenting) | MD (+LD for unplanned stenting) |
| Lee 2008 † | DES (P or S), | 61 | 58 | 9 | LD, MD for 6 mo | LD, MD 200 mg | LD, MD for ≥6 mo |
| Kim 2007 | DES, STEMI | 63 | 70 | 1 | LD 400 mg, MD | LD 300 mg, MD | LD 600 mg, MD |
| Lee 2007 † | DES (P or S), long lesions | 61 | 64 | 1, 9 | LD, MD for 6 mo | LD, MD 200 mg | LD, MD for ≥6 mo |
| Lu 2007 | DES (85%), BMS | 61 | 61 | 6 | MD | LD 100 mg, MD | LD, MD |
| Chen 2006 | BMS | 58 | 62 | 9 | MD for 6 mo | MD indefinitely | MD for 6 mo |
| Douglas 2005 | BMS, angina or silent MI | 60 | 74 | 6 | MD 50 mg twice daily | LD ≥300 mg, MD 1 aspirin | LD 300–600 mg, MD for 1 mo |
⁎ Unless otherwise noted, aspirin, LD 200 mg, MD 100 mg; clopidogrel, LD 300 mg, MD 75 mg; cilostazol, LD 200 mg, MD 100 mg twice daily; unless otherwise noted, each medication was continued for at least the duration of the longest follow-up period. Lee 2011 and Douglas 2005 studies were placebo controlled.
† Lee 2010 reported long-term follow-ups of both Lee 2007 and Lee 2008.
None of the included RCTs were found to have a high risk of bias. Two were found to have a low risk of bias, and 10, an unclear risk. The main reasons for the unclear risk were unclear descriptions of allocation concealment and blinding of participants, personnel, and outcomes assessors. Inspection of our funnel plots revealed no publication bias for any of the pooled outcomes.
Follow-up durations were stratified into short-term (1-month), midterm (1- to 12-month), and long-term (≥12-month) periods. No studies reported outcomes for 1 to 6 months; thus, the midterm follow-up stratum ultimately encompassed 6 to 12 months. Data on major clinical outcomes were extracted ( Table 2 ) and pooled. Compared to DAT, we did not find that the addition of cilostazol led to a significant reduction in TLR at short-term follow-up (2 RCTs, n = 1,103, RR 0.61, 95% CI 0.03 to 12.33; Figure 2 ) . However, compared to the DAT group, the group with additional cilostazol was significantly less likely to experience TLR at midterm follow-up (6 RCTs, n = 3,037, RR 0.57, 95% CI 0.39 to 0.84). Heterogeneity was mild in both short- and midterm follow-up (I 2 = 47.4%, p = 0.168 and I 2 = 24.1%, p = 0.253, respectively). The single study that reported long-term follow-up found lower TLR rates in the group that had received adjunctive cilostazol (n = 900, RR 0.46, 95% CI 0.27 to 0.79).
| Study | Patients (n) | TLR | TVR | MI | Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAT | DAT | TAT | DAT | TAT | DAT | TAT | DAT | TAT | DAT | |
| Short-term (1 month) | ||||||||||
| Han 2009 ⁎ | 604 | 608 | NR | NR | 2 (0.3%) | 3 (0.5%) | 2 (0.3%) | 3 (0.5%) | 3 (0.5%) | 13 (2.1%) |
| Kum 2009 ⁎ | 302 | 301 | 0 | 3 (1.0%) | NR | NR | 0 | 2 (0.7%) | 2 (0.7%) | 2 (0.7%) |
| Kim 2007 | 30 | 30 | NR | NR | 0 | 0 | 2 (6.7%) | 1 (3.3%) | 0 | 0 |
| Lee 2007 ⁎ | 250 | 250 | 1 (0.4%) | 0 | 1 (0.4%) | 0 | 1 (0.4%) | 0 | 0 | 0 |
| Midterm (6–12 months) | ||||||||||
| Lee 2011 | 250 | 249 | 13 (5.2%) | 25 (10%) | 13 (5.2%) | 26 (10.4%) | 4 (1.6%) | 4 (1.6%) | 6 (2.4%) | 3 (1.2%) |
| Suh 2011 | 457 | 458 | 30 (6.6%) | 32 (7.0%) | NR | NR | 4 (0.9%) | 3 (0.7%) | 4 (0.9%) | 6 (1.3%) |
| Han 2009 ⁎ | 604 | 608 | NR | NR | 47 (7.8%) | 63 (10.4%) | 2 (0.3%) | 4 (0.7%) | 16 (2.6%) | 25 (4.1%) |
| Kum 2009 ⁎ | 302 | 301 | 5 (1.7%) | 7 (2.3%) | NR | NR | 1 (0.3%) | 2 (0.7%) | 2 (0.7%) | 3 (1.0%) |
| Kim 2008 | 56 | 53 | NR | NR | 1 (1.8%) | 3 (5.7%) | 1 (1.8%) | 1 (1.9%) | 0 | 0 |
| Lee 2008 † | 200 | 200 | 5 (2.5%) | 14 (7.0%) | 7 (3.5%) | 16 (8.0%) | 1 (0.5%) | 1 (0.5%) | 1 (0.5%) | 0 |
| Lee 2007 ⁎ † | 250 | 250 | 7 (2.8%) | 17 (6.8%) | 9 (3.6%) | 18 (7.2%) | 1 (0.4%) | 1 (0.4%) | 0 | 2 (0.8%) |
| Lu 2007 | 201 | 201 | NR | NR | 13 (6.5%) | 32 (15.9%) | 1 (0.5%) | 3 (1.5%) | 0 | 2 (1.0%) |
| Chen 2006 | 60 | 60 | 3 (5.0%) | 10 (16.7%) | NR | NR | NR | NR | NR | NR |
| Douglas 2005 | 354 | 351 | NR | NR | 54 (15.3%) | 56 (15.9%) | 13 (3.7%) | 10 (2.8%) | 3 (0.8%) | 2 (0.6%) |
| Long-term (24 months) | ||||||||||
| Ahn 2010 | 64 | 66 | NR | NR | 5 (7.8%) | 5 (7.6%) | 1 (1.6%) | 2 (3.0%) | 1 (1.6%) | 2 (3.0%) |
| Lee 2010 † | 450 | 450 | 19 (4.2%) | 41 (9.1%) | 28 (6.2%) | 45 (10.0%) | 4 (0.9%) | 2 (0.4%) | 5 (1.1%) | 6 (1.3%) |
⁎ Han 2009, Kum 2009, and Lee 2007 reported both short- and midterm outcomes.
† Lee 2010 reported long-term follow-ups of both Lee 2007 and Lee 2008.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


