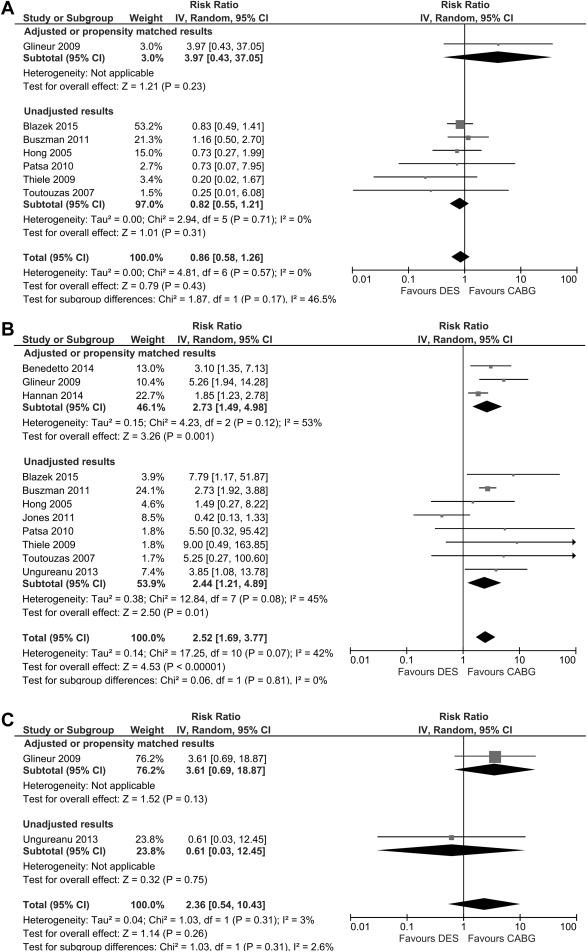
We performed a meta-analysis of the studies comparing the efficacy and safety of coronary artery bypass surgery against percutaneous coronary intervention with drug-eluting stents (PCI-DES) in patients with isolated LAD disease. Because of the limited randomized trial data, the optimal revascularization strategy for patients with isolated LAD disease remains uncertain. Using MEDLINE and EMBASE to source data, 11 studies (3 randomized trials and 8 cohort studies) including 5,044 participants were identified. No significant difference in mortality between PCI-DES and coronary artery bypass surgery (CABG; 111 of 2,122 [5.2%] and 120 of 2,574 [4.7%]; relative risk [RR] 1.23; 95% confidence interval [CI] 0.90 to 1.69) was detected. For MACE, PCI-DES was associated with significant increase in adverse events (RR 1.41; 95% CI 1.03 to 1.93, 8 studies, 4,230 participants). There were no significant differences in the risk of myocardial infarction (RR 0.86; 95% CI 0.58 to 1.26) or stroke (RR 2.36; 95% CI 0.54 to 10.43) between the 2 groups. There were 239 target vessel revascularization (TVR) events among 2,237 participants in the PCI-DES group (10.7%) and 145 TVR events among 2,793 participants in the CABG group (5.2%) with a significant increased risk of TVR in the PCI group (RR 2.52; 95% CI 1.69 to 3.77, 5,030 participants) compared with CABG. In conclusion, for patients with isolated disease of the LAD, meta-analysis of the available data suggests revascularization with a PCI-DES strategy offers similar mortality, MI, and stroke rates to CABG at the expense of increased TVR. Much of the data are derived from registries using first-generation DES, and further randomized trials with more contemporary platforms are needed.
The optimal revascularization strategy for patients with isolated disease of the LAD remains uncertain, in part because of the relative lack of high-quality published reports. At the current time, there are only a small number of randomized control trials of percutaneous coronary intervention with drug-eluting stents (PCI-DES) versus coronary artery bypass surgery (CABG) with small sample sizes and divergent results reported. Consequently, most data in support of the guidelines and best practice are derived from a modest number of small registries and are thus subject to the usual limitations of observational study. Only a single study has been large enough to allow propensity matching in an effort to correct for these confounders and demonstrated a significant excess of repeat revascularization with PCI.
Two small meta-analyses comparing PCI-DES versus CABG have been published previously with inconsistent results reported. One meta-analysis pooled results from 2 randomized controlled trials, whereas a second undertook a subgroup analysis of 5 studies in which outcomes for PCI-DES were compared with minimally invasive direct coronary artery bypass (MIDCAB). Since the publication of these previous meta-analyses, several other registries and one small randomized controlled trial have reported. Therefore, the aim of the present study was to provide an updated review of the literature comparing the efficacy of coronary artery bypass graft surgery with that of PCI-DES in patients with isolated proximal left anterior descending artery disease.
Methods
We searched MEDLINE and EMBASE on October 2015 using the search terms: (drug eluting stent or DES or percutaneous coronary intervention or PCI) AND (bypass or coronary artery bypass graft or CABG or left internal mammary artery or LIMA) AND (left anterior descending or LAD). The search results were reviewed by 2 independent judicators (CSK and AN) for studies that met the inclusion criteria and relevant reviews. The studies identified were validated by MAM. The bibliographies of included studies and relevant reviews were screened for additional studies.
We included studies that met the following inclusion criteria:
- 1.
Studies had to have ≥2 groups with 1 group having PCI with DES and the other having CABG in participants with isolated proximal LAD disease.
- 2.
Studies had to evaluate one or more of the following outcomes: mortality, myocardial infarction, target vessel revascularization (TVR), stroke, or a composite cardiovascular outcome including some or all the above end points.
We excluded studies that had multivessel disease, studies where PCI was undertaken using bare metal stents, and reviews with no original data. Data were extracted from each study and the data collected included the study design, country, year of study, number of participants, age of participants, percentage of male participants, participant selection criteria, stent type, operation, outcomes, follow-up, and results. Quality assessment of the included studies was performed by considering whether the study was prospective, whether the study had >100 participants in each treatment arm, whether there were reliable ascertainment of outcomes, whether there was low loss to follow-up, whether the randomized studies had no baseline differences between arms, and cohort studies has adjusted for confounders.
Data analysis was performed using Review Manager (version 5.3.3; Nordic Cochrane Center, Copenhagen, Denmark) to perform random-effects meta-analysis using generic inverse variance method. Analysis was stratified by whether the analysis used adjustment for potential confounders or propensity score matching or the studies were unadjusted. Where there were asymmetric 95% confidence intervals for reported estimates, we used the absolute larger interval to generate the overall estimate in the pooled results. The I 2 statistic was used to assess statistical heterogeneity.
Results
Our search yielded 1,617 relevant reports, and after screening and reviewing full manuscripts, 11 studies met the inclusion criteria that included 3 randomized trials. The process of study selection is shown in Figure 1 .
The study designs and participant characteristics of the included trials are listed in Table 1 . There were 3 randomized trials and 8 cohort studies. There were a total of 5,044 participants (range of participants in each study 129 to 1,430) with an average age of 63 years and 72% were male participants. Supplementary Table 1 lists the quality assessment of included studies. There were 3 randomized trials and 4 retrospective cohort studies, but the designs of the remaining 4 studies were unclear. Most studies had >100 participants in each arm except for 4 studies (Blazek et al, Hong et al, Thiele et al, and Ungureanu et al ). Five studies reported reliable methods for ascertaining outcomes (Benedetto et al, Blazek et al, Hong et al, Thiele et al, and Toutouzas et al ), whereas the remaining studies did not report how outcomes were ascertained. All 3 randomized trials had similar baseline characteristics among participants and 4 other studies reported some forms of adjustments or propensity score matching.
| Study ID | Design | Country | Year | Total no. of participants, (% male) | Mean age | Participant selection criteria |
|---|---|---|---|---|---|---|
| Benedetto 2014 | Matched cohort study | UK | 2001 to 2013 | 606 (83%) | Unclear | MIDCAB or DES for isolated proximal LAD disease |
| Blazek 2015 | RCT | Germany | 2003 to 2014 | 129 (70%) | 66 | Myocardial ischemia and isolated proximal LAD lesion |
| Buszman 2011 | Retrospective cohort study | Poland | 2004 to 2009 | 463 (75%) | 61 | MIDCAB or DES for isolated proximal LAD disease |
| Glineur 2009 | Retrospective cohort study | Belgium | Unclear | 350 (unclear) | 63 | MIDCAB or DES for isolated proximal LAD disease |
| Hannan 2014 | Cohort study | USA | 2008 to 2011 | 1,430 (66%) | Unclear | CABG or DES for isolated proximal LAD disease |
| Hong 2005 | RCT | South Korea | 2003 | 189 (64%) | 61 | MIDCAB or DES for isolated proximal LAD disease |
| Jones 2011 | Cohort study | England | 2003 to 2010 | 874 (unclear) | Unclear | CABG or DES for isolated proximal LAD disease |
| Patsa 2010 | Cohort study | Greece | Unclear | 412 (unclear) | Unclear | LIMA grafting or DES for isolated proximal LAD disease |
| Thiele 2009 | RCT | Germany | 2003 to 2007 | 130 (70%) | 66 | Isolated stenosis of the LAD |
| Toutouzas 2007 | Retrospective cohort study | Greece | 2001 to 2006 | 257 (86%) | 61 | LIMA or DES revascularization for proximal LAD lesion |
| Ungureanu 2013 | Retrospective cohort study | Belgium | Unclear | 204 (unclear) | Unclear | Revascularization of isolated LAD disease |
Study outcomes are listed in Table 2 . The risk of mortality with PCI-DES compared with CABG for isolated proximal LAD disease is shown in Figure 2 . There were a total of 111 deaths among 2,122 participants (5.2%) who received PCI with PCI-DES and 120 deaths among 2,574 participants (4.7%) who received CABG from 9 studies. The pooled results suggest no significant difference in mortality comparing PCI-DES with CABG for both adjusted and unadjusted results.
| Study ID | Stent | Operation | Follow up and results (MIDCAB/CABG vs DES) |
|---|---|---|---|
| Benedetto 2014 | DES | MIDCAB, LIMA | 2232 days: Deaths 13/303 vs 31/303, HR 2.19 (1.15-4.17). TVR 10 vs 31, HR 3.1 (1.35-4.21). Death/TVR HR 2.14 (1.41-3.24). |
| Blazek 2015 | DES | MIDCAB, LIMA | 7.3 years: Composite 8/65 vs 14/64, RR 1.47 (0.82-2.62). Death 11 vs 9, RR 0.91 (0.58-1.39). MI 6 vs 4, RR 0.83 (0.48-1.41). TVR 1 vs 13, RR 7.79 (1.17-51.87). |
| Buszman 2011 | DES | MIDCAB | 5 years: MACCE 71/276 vs 62/178. Death 12 vs 12. MI 12 vs 9. Revascularization 38 vs 67. |
| Glineur 2009 | DES | MIDCAB | 2 years: MACCE 23/175 vs 65/175, HR 0.33 (0.19-0.55). Death7 vs 7, HR 0.76 (0.25-2.30). MI2 vs 5, HR 0.25(0.03-2.36). Stroke 4 vs 9, HR 0.28 (0.05-1.46). TVR5 vs 26, HR 0.19 (0.07-0.49). |
| Hannan 2014 | DES | – | 3 years: Composite 56/715 vs 46/715, aHR 1.15 (0.76-1.73). Death 39 vs 34, aHR 1.14 (0.70-1.85). Revascularization42 vs 77, aHR 0.54 (0.36-0.81). |
| Hong 2005 | DES | MIDCAB, LIMA | 6 months: Death 2/68 vs 0/116. MI 2 vs 2, RR 0.73 (0.27-1.99). TVR 1vs 3, RR 1.49 (0.27-8.22). |
| Jones 2011 | DES | LIMA graft | 4 years: MACE 15/122 vs 89/752. Death 10 vs 32. TVR 3 vs 44. |
| Patsa 2010 | DES | LIMA graft | 26 months: Death 2/110 vs 5/302. MI 1 vs 2. Target lesion revascularization 0 vs 7. |
| Thiele 2009 | DES | MIDCAB, LIMA | 12 months: MACE 5/65 vs 5/65. MI 5 vs 1. TVR 0 vs 4. |
| Toutouzas 2007 | DES | CABG, LIMA | 18 months: MACE 3/110 vs 4/147. Death 2 vs 3. MI 1 vs 0. TVR 0 vs 3. |
| Ungureanu 2013 | DES | MIDCAB | 2 years: TVR 4/154 vs 5/50. Stroke 2 vs 0. |
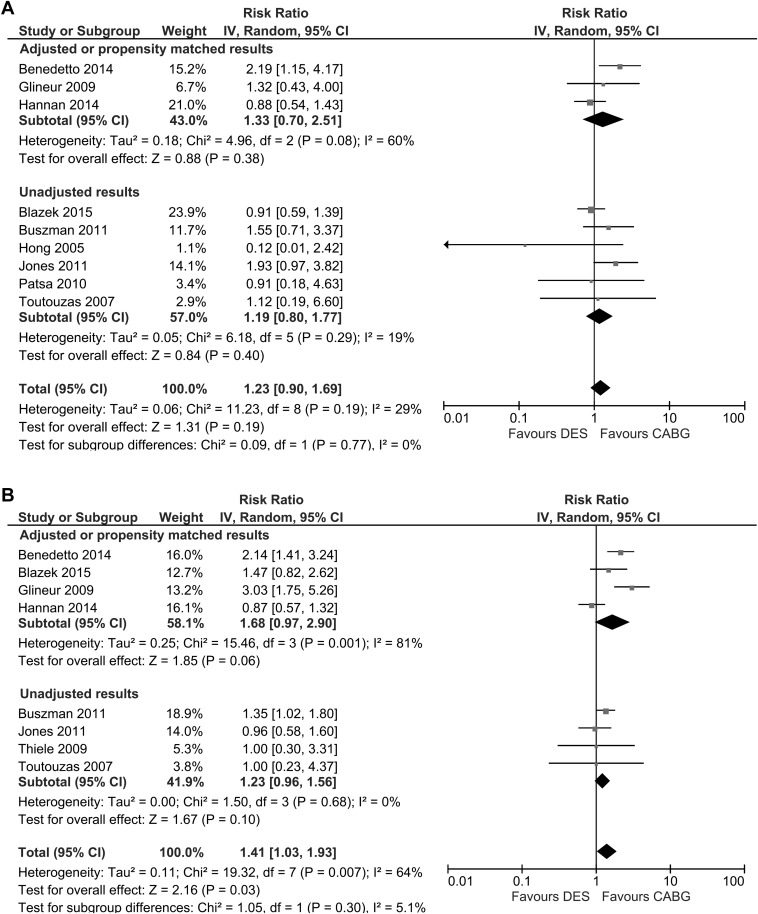
For major adverse cardiovascular events ( Figure 2 ), PCI-DES was not associated with a significant increase in adverse events with adjustments (relative risk [RR] 1.68; 95% confidence interval [CI] 0.97 to 2.90, 4 studies, 2,515 participants), but there was a statistically significant increase in adverse events overall (RR 1.41; 95% CI 1.03 to 1.93, 8 studies, 4,230 participants). For myocardial infarction, treatment with PCI-DES was associated with 23 events among 1,047 participants (2.2%), whereas patients who received CABG had 29 events in 869 participants (3.3%). The pooled results suggest no significant difference in myocardial infarction rates between PCI-DES and CABG for both adjusted and unadjusted results ( Figure 3 ).