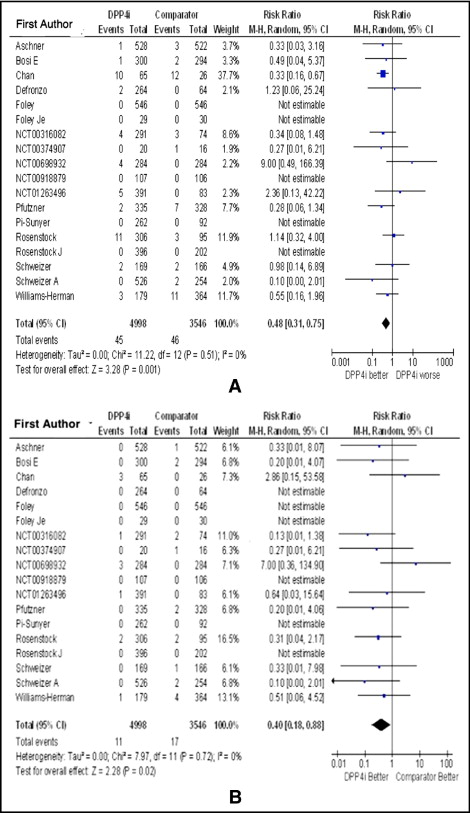
Patients with type 2 diabetes mellitus (DM) have a very high risk for major adverse cardiovascular (CV) events. Previous studies have shown that traditional oral diabetic medications, despite lowering blood glucose levels, generally do not improve CV outcomes. The safety of some oral hypoglycemic medications has been questioned. We aimed to evaluate the CV safety of dipeptidyl peptidase-4 (DPP4) inhibitors, a novel class of oral diabetic medications, by performing a meta-analysis of DPP4 inhibitors for type 2 DM. A search of electronic databases of published and unpublished literature (until September 30, 2011) was performed to identify randomized controlled trials of ≥24 weeks that compared DPP4 inhibitors to other oral diabetic medications. A meta-analysis was performed using fixed and random effects to determine risk ratio (RR) for adverse CV events with DPP4 inhibitor monotherapy compared to other oral diabetic medications or to placebo. Eighteen randomized met our inclusion criteria, comprising 4,998 patients who were randomized to DPP4 inhibitors and 3,546 to a comparator, with a median duration of therapy of 46.4 weeks. In pooled analysis, the RR of any adverse CV event with a DPP4 inhibitor was 0.48 (0.31 to 0.75, p = 0.001), and the RR for nonfatal myocardial infarction or acute coronary syndrome was 0.40 (0.18 to 0.88, p = 0.02). In conclusion, this meta-analysis provides evidence that DPP4 inhibitors are safe from a CV standpoint and may possibly decrease risk of adverse CV events.
Dipeptidyl peptidase-4 (DPP4) inhibitors constitute a new class of agents for treatment of type 2 diabetes mellitus (DM) that has been endorsed by the American Diabetes Association and European Association for the Study of Diabetes as potential first-line therapy for pharmacotherapeutic management of type 2 DM. These drugs are very well tolerated with few side effects and a low incidence of hypoglycemia. The impact of DPP4 inhibitors on long-term cardiovascular (CV) events is being studied in prospective phase III clinical trials; awaiting the results of these trials, it is vital to establish the CV safety of DPP4 inhibitors. The aim of this meta-analysis was to determine the effects of DPP4 inhibitors on CV events by analyzing all relevant randomized controlled trials (RCTs) of patients with type 2 DM treated with DPP4 inhibitor monotherapy versus other oral hypoglycemic agents or placebo.
Methods
This meta-analysis was designed to evaluate the CV safety of DPP4 inhibitors in patients with type 2 DM. We adhered to the recommendations set forth by the Quality of Reporting of Meta-analyses statement for improving the quality of meta-analyses.
A computerized literature search was performed to identify RCTs comparing DPP4 inhibitors to other oral hypoglycemic agents for treatment of type 2 DM. MEDLINE (from 1980 to September 2011), Cochrane Collaborative database, Scopus ( http://www.scopus.com ), and online databases of clinical trials ( http://www.ClinicalTrials.gov ) were searched for relevant journal articles. We also manually searched the references of cited articles and proceedings of relevant national and international meetings for unpublished pertinent material.
The following search terms were used: “dipeptidyl peptidase-4 inhibitors,” “DPP4i,” the names of individual available DPP4 inhibitors (“alogliptin,” “dutogliptin,” “linagliptin,” “saxagliptin,” “sitagliptin,” and “vildagliptin”), and “gliptins.” The search was restricted to articles published in the English language. Abstracts of cited articles were reviewed by 2 independent investigators (H.R.P. and F.J.A.) to determine relevance. Studies were retrieved for further consideration if judged pertinent by 1 reviewer or 2 reviewers. Discrepancies were identified and resolved by consensus or, as needed, with a third investigator and confirmed by consensus. When an abstract from a meeting and a full article referred to the same trial, only the full article was included in the analysis. When there were multiple reports from the same trial, we used the most complete and/or more recently reported data.
We included studies in this meta-analysis if (1) they were RCTs comparing DPP4 inhibitor monotherapy in 1 arm to other oral antitype 2 DM agents (other than DPP4 inhibitors) in the comparator arm, (2) duration of treatment was ≥24 weeks, and (3) reported data on adverse CV side effects of DPP4 inhibitor therapy (death from CV causes, nonfatal myocardial infarction or acute coronary syndrome, stroke, heart failure, and arrhythmias). The quality of included articles was further assessed using Jadad criteria.
Extracted data included study authors, total number of patients in each arm, drug and dose used in DPP4 inhibitor and comparator arms, and incidence of adverse CV events (death, nonfatal myocardial infarction or acute coronary syndrome, and stroke). Two reviewers performed independent data extraction (H.A.A. and S.K.B.), all discrepancies were identified and resolved by consensus or, as needed, with a third investigator (HRP) and confirmed by consensus. When a study design included >2 arms (e.g., when comparing different doses of a DPP4 inhibitor), all patients treated with DPP4 inhibitor only were included in the intervention arm irrespective of dose, and patients treated with placebo or oral hypoglycemic agents (other than DPP4 inhibitors) were included in the comparator arm.
The primary end point was development of adverse CV events defined as death from CV causes, nonfatal myocardial infarction or acute coronary syndrome, stroke and arrhythmias (including atrial fibrillation, supraventricular tachycardia, or palpitations), and heart failure. Secondary end points included individual components of the primary end point whenever adequate data were reported to calculate effect size.
We calculated risk ratios (RRs) for developing adverse CV events using fixed- and random-effect models. We reported only the summary effect size derived using the fixed-effect model unless significant heterogeneity was observed, at which time effect size computed using the random effect model was reported. The RR for adverse CV events was retrieved with the 95% confidence interval (CI). Quantitative analyses were performed on an intention-to-treat basis and were confined to data derived from period of follow-up. We planned to perform subgroup analyses of the primary end point a priori. For these analyses, studies were grouped by (1) individual DPP4 inhibitor agents, (2) intervention in the comparator arm (placebo, metformin, sulfonylurea, or thiazolidinediones), and (3) duration of therapy (≤52 or >52 weeks).
Heterogeneity between studies was analyzed as I 2 = ([Q − df]/Q) × 100%, where Q is the chi-square statistic and df is its degrees of freedom. This describes the percent variability in effect estimates that is due to heterogeneity rather than sampling error (chance). A value >50% was considered to indicate substantial heterogeneity. Publication bias was assessed graphically using an inverted funnel plot.
All analyses were performed with RevMan Analyses 5.1.4 (Review Manager, Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark).
Results
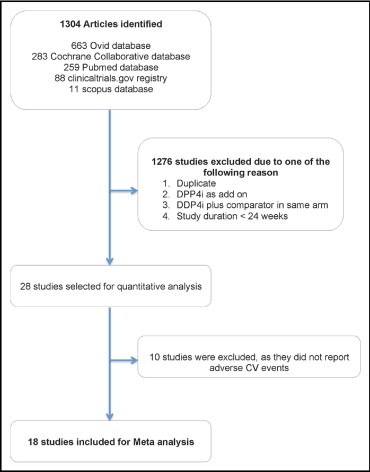
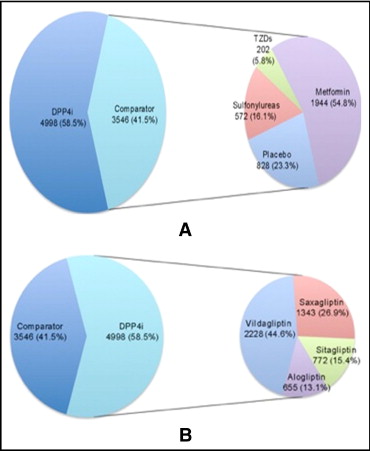
Of the 1,304 citations identified initially, 28 full-text articles were reviewed for relevance. Ten RCTs that examined the efficacy of DPP4 inhibitors but did not report data on adverse CV events, including 5,631 patients, were excluded. Eighteen nonoverlapping RCTs met our inclusion criteria ( Figure 1 ). Pooled analysis included 8,544 patients (4,998 randomized to a DPP4 inhibitor and 3,546 to placebo or other oral hypoglycemic agents). Of patients randomized to receive DPP4 inhibitor therapy, 2,228 patients (44.6%) were treated with vildagliptin, 1,343 (26.9%) with saxagliptin, 772 with sitagliptin (15.4%), and 655 (13.1%) with alogliptin. No studies of dutogliptin or linagliptin met our inclusion criteria, so none were included in the final analysis. In the comparator arm, 1,944 patients were randomized to metformin (54.8%), 828 to placebo (23.3%), 572 to sulfonylureas (16.1%), and 202 to thiazolidinediones (5.8%; Figure 2 ). Of included studies, 13 were international multicenter trials and 2 were conducted in North America and 3 in Asia.
Overall, included studies were of adequate methodologic quality (mean Jadad score 3.5 for included studies, 14 of 20 studies had a score ≥3). Included studies, basic characteristics of enrolled patients, and details about drug therapy in the 2 arms are presented in Table 1 .
| Study | Region | Mean Age (years) | Men | DPP4 Inhibitor | Dose (mg) | Comparator | Duration (weeks) | Study Size | Follow-Up (person-years) | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Bosi et al | multiregional | 53 | 59% | vildagliptin | 50 | metformin | 24 | 1,179 | 28,296 | 2 |
| DeFronzo et al | multiregional | 53 | 53% | alogliptin | 12.5, 25 | placebo | 26 | 329 | 8,554 | 5 |
| Pfützner et al | multiregional | 52 | 50% | saxagliptin | 10 | metformin | 76 | 1,306 | 99,256 | 4 |
| Williams-Herman et al | multiregional | 54 | 50% | sitagliptin | 100 | metformin | 104 | 1,091 | 113,464 | 5 |
| Aschner et al | multiregional | 56 | 48% | sitagliptin | 100 | metformin | 24 | 1,050 | 25,200 | 5 |
| Foley and Sreenan | multiregional | 55 | 59% | vildagliptin | 50 | sulfonylureas | 104 | 1,092 | 113,568 | 4 |
| Rosenstock et al | multiregional | 53 | 51% | saxagliptin | 2.5, 5, 10 | placebo | 24 | 401 | 9,624 | 5 |
| Schweizer et al | multiregional | 71 | 48% | vildagliptin | 100 | metformin | 24 | 335 | 8,040 | 4 |
| Chan et al | multiregional | 69 | 48% | sitagliptin | 25, 50, 100 | sulfonylureas | 54 | 91 | 4,914 | 4 |
| Schweizer et al | multiregional | 53 | 54% | vildagliptin | 100 | metformin | 52 | 780 | 40,560 | 3 |
| Pi-Sunyer et al | multiregional | 51 | 56% | vildagliptin | 50, 100 | placebo | 24 | 354 | 8,496 | 3 |
| Rosenstock et al | multiregional | 54 | 55% | vildagliptin | 50 | TZD | 104 | 598 | 62,192 | 5 |
| Foley et al | North America | 57 | 58% | vildagliptin | 100 | placebo | 52 | 59 | 3,068 | 3 |
| Unpublished | Asia | 61 | 72% | alogliptin | 6.25, 12.5, 25, 50 | voglibose, placebo | 52 | 474 | 24,648 | 2 |
| Unpublished | multiregional | 55 | 44% | saxagliptin | 2.5, 5 | placebo | 24 | 365 | 8,760 | 2 |
| Unpublished | North America | 55 | 39% | saxagliptin | 5 | metformin, placebo | 116 | 36 | 4,176 | 2 |
| Unpublished | Asia | 51 | 55% | saxagliptin | 5 | placebo | 24 | 568 | 13,632 | 2 |
| Unpublished | Asia | 48 | 56% | saxagliptin | 5 | placebo | 24 | 213 | 5,112 | 2 |
Overall, use of DPP4 inhibitors was associated with a lower risk of adverse CV effects (RR 0.48, 95% CI 0.31 to 0.75, p = 0.001; Figure 3 ) and a lower risk of nonfatal myocardial infarction or acute coronary syndrome (RR 0.40, 95% CI 0.18 to 0.88, p = 0.02) compared to placebo or other oral hypoglycemic agents ( Figure 3 ). Subgroup analysis by the studied DPP4 inhibitors showed a significantly lower risk of adverse CV events with sitagliptin (RR 0.37, 95% CI 0.21 to 0.68, p = 0.001) but not with saxagliptin (RR 0.64, 95% CI 0.23 to 1.76, p = 0.39), alogliptin (RR 1.73, 95% CI 0.21 to 13.93, p = 0.61), or vildagliptin (RR 0.50, 95% CI 0.13 to 1.92, p = 0.31; Figure 4 ). Risk of adverse CV events with DPP4 inhibitor therapy was not significantly different compared to placebo (RR 1.05, 95% CI 0.39 to 2.82, p = 0.92) but was significantly lower compared to metformin (RR 0.42, 95% CI 0.20 to 0.87, p = 0. 02) and other oral hypoglycemic agents including sulfonylureas and thiazolidinediones (RR 0.33, 95% CI 0.16 to 0.67, p = 0.002; Figure 4 ). In addition, studies with a duration of ≥52 weeks demonstrated a lower risk of adverse CV events with DPP4 inhibitor treatment compared to control (RR 0.37, 95% CI 0.21 to 0.63, p = 0.0003), which was not seen in the subset of studies with <52 weeks of DPP4 inhibitor therapy (RR 0.78, 95% CI 0.38 to 1.60, p = 0.50; Figure 5 ). Analysis without including studies that had no events in the 2 arms showed no change in effect size; the RR of major adverse CV side effects was 0.48 (95% CI 0.31 to 0.75, p = 0.001). In analysis without including sitagliptin studies, the RR for major adverse CV side effects with other DPP4 inhibitors was 0.65 (95% CI 0.35 to 1.23, p = 0.19) compared to placebo or other oral hypoglycemic agents.