Little is known about transcatheter aortic valve implantation (TAVI) in patients with bicuspid aortic valve stenosis, which usually represents a contraindication. The aim of this study was to assess the feasibility and the results of TAVI in this patient subset. Of 316 high-risk patients with severe aortic stenosis who underwent TAVI from January 2009 to January 2012, 15 (5%) had documented bicuspid aortic valves. They were treated using a transarterial approach, using the Medtronic CoreValve system. Patients were aged 80 ± 10 years, in New York Heart Association functional classes III and IV. The mean aortic valve area was 0.8 ± 0.3 cm 2 , and the mean gradient was 60 ± 19 mm Hg. The mean calcium score, calculated using multislice computed tomography, was 4,553 ± 1,872 arbitrary units. The procedure was successful in all but 1 patient. Major adverse events, according to Valvular Academic Research Consortium definitions, were encountered in 1 patient (death). The mean postimplantation prosthetic gradient was 11 ± 4 mm Hg, and ≤1+ periprosthetic leaks were observed in all but 2 patients. The mean prosthetic ellipticity index was 0.7 ± 0.2 at the level of the native annulus and 0.8 ± 0.2 at the level of the prosthetic leaflets. After a mean follow-up period of 8 ± 7 months, 1 patient had died from aortic dissection; there were no additional adverse events. All but 2 hospital survivors were in New York Heart Association class I or II. In conclusion, the present series suggests that transarterial Medtronic CoreValve implantation is feasible in selected patients with bicuspid aortic valve and may lead to short-term hemodynamic and clinical improvement.
Bicuspid aortic valve (BAV) is the most common congenital heart disease and may lead to aortic stenosis (AS). Transcatheter aortic valve implantation (TAVI) has emerged as an attractive alternative to surgery in patients with symptomatic AS and contraindications to surgery or in selected patients at high risk for surgery. However, because of its specific anatomic characteristics, BAV is still considered a contraindication to TAVI, and there have been only a few reported cases of TAVI in patients with BAVs. Here, we describe our experience with TAVI in this specific population, using the Medtronic CoreValve system (MCS; Medtronic, Inc., Minneapolis, Minnesota) and a transarterial approach.
Methods
The study population included patients with documented BAVs who were treated using TAVI with the MCS. Preoperative screening included clinical evaluation, with a specific analysis of the anatomy of the BAV. Otherwise, screening did not differ from standard TAVI. Then, a heart team evaluated the risk profiles of the patients and reached a consensus regarding the method of treatment.
The diagnosis of BAV was based on short-axis imaging of the aortic valve using transthoracic or transesophageal echocardiography. The diagnosis of BAV was confirmed in the presence of only 2 commissures delimiting only 2 aortic leaflets. BAVs were classified on the basis of the presence (type 1) or absence (type 0) of raphe.
AS severity was assessed using the transaortic mean gradient and the aortic valve area. The amount and distribution of calcification were noted. Associated aortic regurgitation (AR) was evaluated according to published guidelines. The aortic annulus was measured at the insertion of the aortic cusps. Multislice computed tomography confirmed the diagnosis and assessed the type of BAV, the amount and distribution of calcification, and the aortic diameters, including the annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta; the calcium score was calculated as previously described. The technical aspects of TAVI using the MCS have been previously described. Procedures were performed using either the retrograde transfemoral or left subclavian approach. TAVI was performed either under local anesthesia with conscious sedation or under general anesthesia with transesophageal echocardiographic guidance. The 18Fr transfemoral arterial access was obtained percutaneously using the Prostar 10 F XL (Abbot Vascular Devices, Redwood City, California). The subclavian procedure was performed using surgical cut-down access and closure. Balloon predilatation was performed using an undersized balloon, usually 20 or 22 mm in diameter.
Postoperatively, patients received a combination of aspirin and clopidogrel for 3 months (then aspirin or clopidogrel alone) or clopidogrel and oral anticoagulation if otherwise indicated.
Clinical follow-up, transthoracic echocardiography, and when possible multislice computed tomography were performed before discharge. Several parameters were specifically assessed: the mitral overlap by the MCS (the distance between the insertion of the anterior mitral leaflet and the ventricular end of the prosthesis), an ellipticity index of the prosthesis (the ratio of small prosthetic diameter to large prosthetic diameter at the levels of the native annulus and of the hinge points of prosthetic leaflets, located 12 mm above the ventricular end of the MCS), and the implantation depth of the prosthesis (the distance between the noncoronary cusp and the ventricular end of the prosthesis). Adverse events were prospectively recorded. Thirty-day, 6-month, and 1-year medical visits and transthoracic echocardiography were performed at our institution or by the patients’ own cardiologists, and data were collected by telephone.
Device success and end point definitions were the standardized definitions of the consensus report from the Valvular Academic Research Consortium.
Data are expressed as mean ± SD or as median (interquartile range). Statistical analysis was performed using JMP version 7.0.1 Statistical Discovery (SAS Institute Inc., Cary, North Carolina).
Results
From January 2009 to January 2012, of the 316 patients who underwent TAVI, 15 (5%) had documented BAVs and received MCS. Patients’ clinical baseline characteristics are listed in Table 1 . The mean age was 80 ± 10 years; their mean Society of Thoracic Surgeons risk score and logistic European System for Cardiac Operative Risk Evaluation score were 8 ± 5% and 17 ± 11%, respectively.
| Patient | Age (years) | Gender | NYHA Class | eGFR <60 ml/min | Co-Morbidities >2 | High Risk for Surgery ⁎ | STS Score | Logistic EuroSCORE |
|---|---|---|---|---|---|---|---|---|
| 1 | 51 | M | III | 0 | 0 | Cancer | 1% | 4% |
| 2 | 73 | M | III | + | + | Porcelain aorta | 9% | 13% |
| 3 | 74 | F | IV | 0 | + | Respiratory insufficiency | 6% | 9% |
| 4 | 75 | M | III | 0 | + | Respiratory insufficiency | 5% | 4% |
| 5 | 76 | M | III | + | + | Cancer | 5% | 7% |
| 6 | 79 | F | III | 0 | 0 | Cognitive dysfunction | 3% | 16% |
| 7 | 79 | M | III | + | + | — | 15% | 27% |
| 8 | 81 | M | III | 0 | + | 12% | 23% | |
| 9 | 84 | F | IV | 0 | 0 | — | 6% | 20% |
| 10 | 85 | M | IV | 0 | + | 12% | 36% | |
| 11 | 86 | M | III | + | + | — | 10% | 23% |
| 12 | 87 | M | III | 0 | + | Cancer | 6% | 18% |
| 13 | 88 | M | IV | + | + | — | 18% | 40% |
| 14 | 89 | M | III | 0 | 0 | Frailty | 7% | 10% |
| 15 | 90 | M | III | 0 | 0 | Frailty | 5% | 10% |
Echocardiographic findings were as follows: mean aortic valve area 0.8 ± 0.3 cm 2 (0.5 ± 0.1 cm 2 /m), mean aortic gradient 60 ± 19 mm Hg, mean left ventricular ejection fraction 52 ± 13%, and mean aortic annular diameter 24 ± 2 mm. Dimensions of the aorta were 25 ± 2, 36 ± 3, 32 ± 4, and 37 ± 3 mm at the level of the annulus, the sinuses of Valsalva, the sinotubular junction, and the ascending aorta, respectively. The presence of a raphe was observed in 10 patients. Two patients had no raphe. The type of BAV was uncertain in 3 patients. Calcifications were qualified as moderate in 7 and severe in 8 and were predominantly located on the raphe in 3 and on the free edges of the cusps in 6 and were diffuse in 6. Associated AR was present in 8 patients (grade 1+ in 4 patients and grade 2+ in 4 patients).
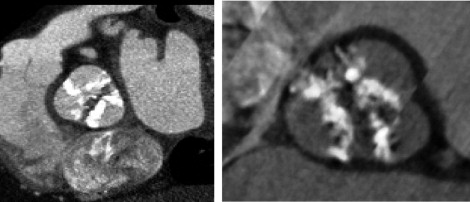
Multislice computed tomographic findings are listed in Table 2 . The mean annular diameter was calculated at 26 ± 2 mm. Mean distances between the aortic annulus and the left and right coronary ostia were 14 ± 3 mm (range 11 to 20) and 15 ± 4 mm (range 8 to 22), respectively. The maximal ascending aortic diameter was 37 ± 4 mm. Overall, findings were consistent with those from echocardiographic imaging. Type 1 BAVs were found in 10 patients and type 0 BAVs in 2 ( Figure 1 ). In 3 cases, the type of BAV was not identifiable, because of extensive valve calcification. The calcium score was 4,553 ± 1,872 arbitrary units.
| Patient | Bicuspid Valve Type ⁎ | Mean Annular Diameter (mm) † | Calcium Score ‡ | Distribution of Calcium | Ascending Aortic Maximal Diameter (mm) | |
|---|---|---|---|---|---|---|
| Raphe | Free Edges | |||||
| 1 | ? | 27 | 3,591 | + | + | — |
| 2 | 1 | 28 | 3,285 | + | 0 | 37 |
| 3 | 1 | 25 | 2111 | 0 | + | 35 |
| 4 | 0 | 26 | — | + | + | 32 |
| 5 | 1 | 25 | 2148 | 0 | + | 40 |
| 6 | 1 | 25 | 5,524 | 0 | + | 40 |
| 7 | 1 | 28 | 5,765 | 0 | + | 37 |
| 8 | 1 | 25 | 4,958 | 0 | + | 37 |
| 9 | ? | 23 | 6,514 | + | + | 41 |
| 10 | 1 | 31 | 6,213 | + | 0 | 39 |
| 11 | 1 | 28 | 5,601 | + | + | 37 |
| 12 | 0 | 25 | 5,458 | 0 | + | 38 |
| 13 | 1 | 29 | 1884 | 0 | + | 31 |
| 14 | ? | 26 | 7,772 | + | + | 39 |
| 15 | 1 | 26 | 2,917 | + | + | 37 |
⁎ Type 0, no raphe; type 1: 1 raphe.
† Average of maximal and minimal annular diameters.
‡ Performed according to Agatston et al and expressed in arbitrary units.
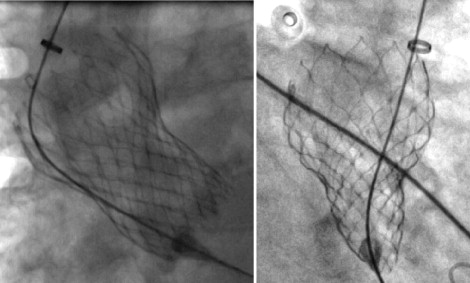
Procedures were performed by transfemoral access in 14 patients and by subclavian access in 1. General anesthesia was used in 3 patients. In the latter, 2-dimensional and 3-dimensional transesophageal echocardiography was useful for guiding prosthesis deployment. The 29-mm MCS was implanted in 13 patients and the 26-mm MCS in 2. The AccuTrack system was used in 9 patients. The procedure was successful in 14 patients. There was no intraprocedural death. In the first patient, the MCS was implanted too low in an extensively calcified BAV, resulting in 3+ paraprosthetic AR; the patient developed acute respiratory distress a few hours after implantation. Emergency surgical aortic valve replacement was carried out, but the patient did not survive. Despite asymmetric expansion of the MCS on fluoroscopy in several patients ( Figure 2 ), immediate hemodynamic performance of the prostheses was satisfactory in 13 patients, with paraprosthetic or central AR grade ≤1+ and transprosthetic gradient <20 mm Hg ( Figure 3 ).
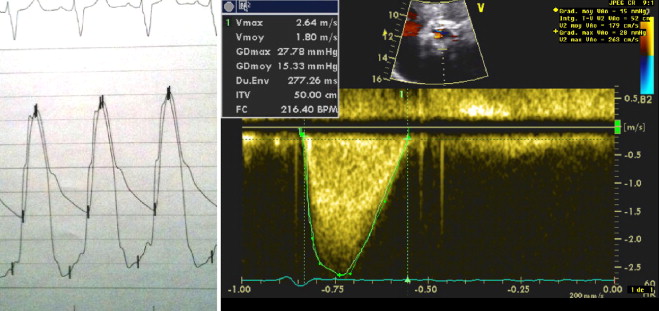
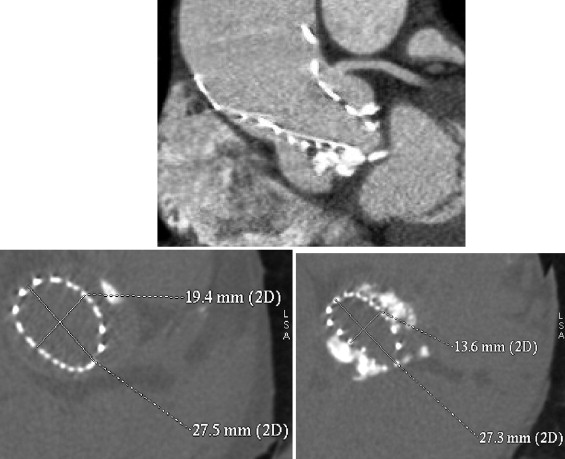
The median hospital stay from procedure to discharge was 9 days (interquartile range 8 to 10). The 30-day combined safety end point rate was achieved in 14 patients (93%). There was 1 in-hospital death, described earlier; there was no other major adverse events. Permanent pacemakers were required in 6 patients. On predischarge transthoracic echocardiography, the mean transprosthetic gradient was 11 ± 4 mm. Mild AR (1+) was observed in 13 patients, and 1 had AR 2+. The mean mitral overlap by the MCS was 6 ± 4 mm, with no impact on the function of the mitral valve. In the 11 patients who underwent post-TAVI multislice computed tomography, mean prosthetic ellipticity indexes were 0.7 ± 0.2 and 0.8 ± 0.2 at the levels of the native annulus and of the prosthetic leaflets, respectively. An ellipticity index <0.8 at the level of the leaflets was observed in only 2 patients: it was 0.7 in patient 12, who had a marked elliptical shape of the prosthesis at the level of the annulus (ellipticity index 0.5; Figure 4 ), and 0.4 in patient 14, who had the most extensive valvular calcification observed in the series, with a calcium score of 7,772 arbitrary units. On average, the implantation depth was 7 ± 5 mm (range 0 to 15). In the 3 patients who had deep-seated MCS (implantation depth >10 mm), there was no difference between the ellipticity indexes calculated at the level of the annulus and of the prosthetic leaflets ( Table 3 ).
| Patient | Transthoracic Echocardiography | |||||||
|---|---|---|---|---|---|---|---|---|
| AR | Computed Tomography | |||||||
| Mean Gradient (mm Hg) | Peak Gradient (mm Hg) | Central | Paravalvular | Mitral Overlap ⁎ | Ellipticity Index, Aortic Annular Level † | Ellipticity Index, Prosthetic Leaflets Level † | Implantation Depth (mm) ‡ | |
| 1 | 16 | 28 | 0 | + | 6 | 0.8 | 0.9 | 7 |
| 2 | 9 | 19 | 0 | + | 8 | 0.8 | 0.9 | 9 |
| 3 | 15 | 26 | 0 | + | 8 | — | — | — |
| 4 | — | — | 0 | + | — | — | — | — |
| 5 | 6 | 10 | 0 | + | — | — | — | — |
| 6 | 9 | 28 | 0 | 0 | 0 | 0.5 | 0.8 | 0 |
| 7 | 8 | 27 | 0 | + | 1 | 0.9 | 1.0 | 2 |
| 8 | 12 | 28 | 0 | + | 7 | 0.8 | 0.9 | 7 |
| 9 | 14 | 23 | 0 | + | 9 | 0.8 | 0.8 | 13 |
| 10 | 5 | 13 | 0 | + | 10 | 0.7 | 0.8 | 7 |
| 11 | 11 | 16 | 0 | + | 6 | 0.9 | 0.9 | 11 |
| 12 | 10 | 32 | + | + | 0 | 0.5 | 0.7 | 0 |
| 13 | 8 | 16 | 0 | + | 7 | — | — | — |
| 14 | 18 | 32 | 0 | + | 5 | 0.4 | 0.4 | 7 |
| 15 | 10 | 26 | 0 | + | 14 | 0.9 | 0.8 | 15 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


