In October 2007, the Federal Drug Agency issued a black box warning for contrast agents used in patients undergoing echocardiography and restricted their use in patients with acute coronary syndrome, a decompensated heart, and respiratory failure. We performed a systemic review and meta-analysis to study the adverse effects of contrast agents used with respect to myocardial infarction and all-cause mortality. MEDLINE, EMBASE, BIOSIS, and Cochrane databases from inception to October 2009 were searched for studies that reported myocardial infarction and all-cause mortality after the use of contrast agents for echocardiography. A total of 8 studies were included in the present meta-analysis. A random-effect model was used, and between-studies heterogeneity was estimated with I 2 . A total of 8 studies reported death as an outcome and only 4 reported myocardial infarction. The incidence of death in the contrast group was 0.34% (726 of 211,162 patients) compared to 0.9% (45,970 of 5,078,666 patients) in the noncontrast group. The pooled odds ratio was 0.57 (95% confidence interval 0.32 to 1.01, p = 0.05). The reported incidence of myocardial infarction in the contrast group was 0.15% (86 of 57,264 patients) compared to 0.2% (92 of 44,503 patients) in the noncontrast group. The pooled odds ratio was 0.85 (95% confidence interval 0.35 to 2.05, p = 0.72). Significant heterogeneity was seen among the studies. In conclusion, the cumulative evidence has suggested that the use of contrast agents for echocardiography is safe and not associated with a greater incidence of myocardial infarction or and mortality.
Although several studies have been published regarding the safety and efficacy of ultrasound contrast agents, a postmarketing review by the Food and Drug Administration (Federal Drug Agency) resulted in the issuance of a black box warning for all ultrasound contrast agents in October 2007. This decision was based on 4 reported deaths that occurred within 30 minutes of the administration of an ultrasound contrast agent. The use of these agents thereby became contraindicated in patients with acute cardiopulmonary syndromes, severe pulmonary hypertension, and QT prolongation. On closer review, however, these events were deemed to be related to the patient’s underlying medical condition; thus, subsequently, the contraindications were lifted from the labeling in May 2008. Despite that and additional publications advocating the safety of ultrasound contrast agents, their cost-effectiveness, and the positive clinical effect of their use, the use of contrast agents in clinical echocardiography has significantly decreased. This has probably been related to the continued generalized “fear” in echocardiography laboratories that these agents are still considered potentially harmful, especially in critically ill patients. We, therefore, conducted a systemic review and meta-analysis of 8 studies reporting the incidence of myocardial infarction (MI) and all-cause mortality as an end point after the administration of contrast agents.
Methods
We conducted a search in the MEDLINE (1948 to November 2009), EMBASE (1988 to 2009, week 40), and Cochrane (from inception to the third quarter of 2009) databases for studies that reported MI and all-cause mortality after the use of contrast agents for echocardiography. We used the following keywords for the search: contrast echocardiography, death and contrast echocardiography, myocardial infarction and echocardiography, and others. The search was performed without any language restrictions but was limited to human subjects. When an abstract from a meeting and a full report referred to the same study, only the full report was included in the analysis. When multiple reports were available from the same study, we used the most complete and/or recently reported data.
We only included the studies that reported MI and/or all-cause mortality after the use of contrast agents for echocardiography at varying intervals. Only studies that compared the adverse event rate for MI and all-cause mortality between 2 groups (i.e., a contrast and noncontrast group) were selected for the present meta-analysis. The studies were also reviewed for the incidence of allergic/anaphylactic reactions in patients receiving the echocardiography contrast agent. The data from each trial was abstracted by an investigator (MA) and was confirmed by a secondary investigator (OK). The studies that did not meet these requirements (ie, reporting MI and death) were excluded from the present meta-analysis.
The meta-analyses were performed by computing the odds ratio (OR) using a random-effects model. The ORs and the 95% confidence intervals (CIs) for MI and death after contrast echocardiography were calculated. Between-study heterogeneity was analyzed using the following equation: I 2 = [(Q − df)/Q] × 100%, where Q was the chi-squared statistic and df was the degrees of freedom. An I 2 >50% indicated heterogeneity. This describes the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error (chance). Publication bias was assessed graphically using a funnel plot. All analyses were performed with RevMan Analyses, version 5.0.20 (Nordic Cochrane Centre, Rigshopitalet, Copenhagen, Denmark).
Results
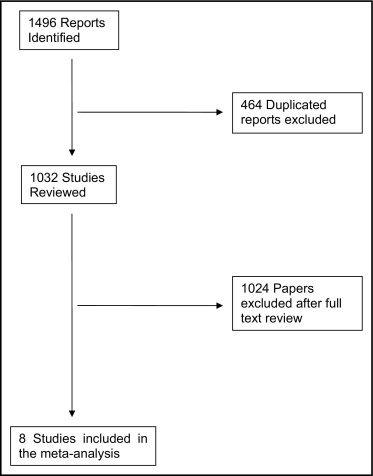
Overall, we found 1,496 reports in the primary search, of which 464 were excluded because of duplication. Of the remaining 1,032 reports, 8 were included in total after a full text review. Figure 1 shows the results of the literature search. Of the 8 included studies, 8 reported death as an outcome but only 4 also reported MI as a prespecified end point. The basic characteristics of these studies are listed in Table 1 .
| Abdelmoneim et al | Gabriel et al | Dolan et al | Shaikh et al | Main et al | Wei et al | Kusnetzky et al | Anantharam et al | |
|---|---|---|---|---|---|---|---|---|
| Patients (n) | 26,774 | 9,798 | 66,220 | 5,069 | 4,300,966 | 858,626 | 18,671 | 3,704 |
| Contrast | 10,792 | 4,786 | 42,408 | 2,914 | 58,254 | 78,383 | 12,475 | 1,150 |
| No contrast | 15,982 | 5,012 | 23,812 | 2,155 | 4,242,712 | 780,243 | 6,196 | 2,554 |
| Population type | NA | Outpatient mostly | Outpatients and inpatients | Outpatients | Hospitalized patients | Outpatients and inpatients | Hospitalized patients | Hospitalized patients |
| Outcomes recorded | Death at 72 h and 30 d MI at 72 h and 30 d | Death at 24 h and 30 d | Death at 30 min, 24 h, and 30 d MI at 30 min, 24 h, and 30 d | Death and MI during stress testing | Death at 24 h | Death at 30 min | Death at 24 h | Death and MI |
| Age (years) | ||||||||
| Contrast | 66 | 61 | NA | 61 | 67 | 60 | 66 | 64.5 |
| No contrast | 63 | 60 | 58 | 70 | 59 | 64 | 64 | |
| Ejection fraction (%) | ||||||||
| Contrast | 59% | 54% | NA | NA | NA | NA | NA | NA |
| No contrast | 60% | 55% | ||||||
| Men | ||||||||
| Contrast | 57% | 64% | 64% | 53% | 61% | 49% | 64% | 57% |
| No contrast | 53% | 55% | 59% | 51% | 47% | 63% | 42% | 53% |
| Body mass index (kg/m 2 ) | ||||||||
| Contrast | 31 | NA | NA | 28 | NA | 32 | NA | NA |
| No contrast | 27 | 26 | 28 | |||||
| Hypertension | ||||||||
| Contrast | 68% | NA | 57% | 36% | NA | NA | 86% | 45% |
| No contrast | 54% | 53% | 23% | 59% | 44% | |||
| Diabetes mellitus | ||||||||
| Contrast | 26% | NA | 35% | 17% | NA | NA | 36% | 28% |
| No contrast | 15% | 30% | 9% | 18% | 18% | |||
| Dyslipidemia | ||||||||
| Contrast | 68% | NA | 51% | 19% | NA | NA | NA | 35% |
| No contrast | 58% | 52% | 10% | 28% |
Studies reporting all-cause mortality (i.e., the ones conducted by Abdelmoneim et al, Anantharam et al, Dolan et al, Gabriel et al, Kusnetzky et al, Shaikh et al, Main et al, and Wei et al ) had a total of 5,289,828 patients, with 211,162 patients (4%) in the contrast group and 5,078,666 (96%) in the noncontrast group. In the latter group, Main et al had the most patients (4,300,966 [81%]) and Anantharam et al had the fewest patients (3,704 [0.07%]). Although all 8 included studies provided data to permit the calculation of the mortality rates in the contrast and noncontrast groups, only 5 contributed to the calculation of the corresponding OR. Similarly, the funnel plot assessment for publication bias was also limited to 5 of the 8 studies. The cumulative incidence of all-cause mortality in the contrast group was 0.34% (726 of 211,162 patients) compared 0.9% (45,970 of 5,078,666 patients) in the noncontrast group. The pooled OR was 0.57 (95% CI 0.32 to 1.01, p = 0.052; Figure 2 ).
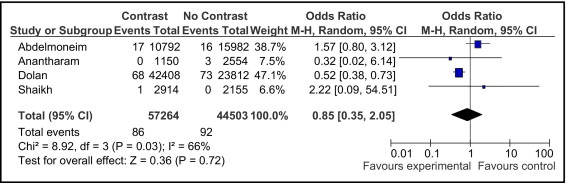
The incidence of MI was reported in 4 of the 8 studies. These studies (i.e., the ones conducted by Abdelmoneim et al, Anantharam et al, Dolan et al, and Shaikh et al ) included 101,767 patients, with 57,264 (56%) in the contrast group and 44,503 (44%) in the noncontrast group. The incidence of MI was 0.15% (86 of 57,264 patients) in the contrast group compared to 0.2% (92 of 44,503 patients) in the noncontrast group. The pooled OR was 0.85 (95% CI 0.35 to 2.05, p = 0.72; Figure 3 ).
We also calculated the ORs for all-cause mortality, after excluding each study one by one, which was then compared with the pooled OR for all-cause mortality by performing a sensitivity analysis. These ORs were found to be statistically insignificant ( Table 2 ). Likewise, the OR was calculated for MI after excluding each study that reported the incidence of MI one by one, which was then compared with the pooled OR for MI by performing a sensitivity analysis. The differences were statistically insignificant ( Table 3 ). The results were also adjusted for studies reporting death or MI at 30 minutes, 24 hours, and 30 days, which were then compared with the pooled OR for death or MI, respectively, using a sensitivity analysis and again were statistically insignificant.
| Variable | Adjusted OR | p Value |
|---|---|---|
| Excluding Wei et al | 0.57 | 1.0 |
| Excluding Shaikh et al | 0.57 | 1.0 |
| Excluding Main et al | 0.49 | 0.624 |
| Excluding Kusnetzky et al | 0.69 | 0.536 |
| Excluding Gabriel et al | 0.56 | 0.954 |
| Excluding Dolan et al | 0.66 | 0.635 |
| Excluding Anantharam et al | 0.57 | 1.0 |
| Excluding Abdelmoneim et al | 0.50 | 0.671 |
| Variable | Adjusted OR | p Value |
|---|---|---|
| Excluding Shaikh et al | 0.79 | 0.812 |
| Excluding Dolan et al | 1.48 | 0.072 |
| Excluding Anantharam et al | 0.93 | 0.771 |
| Excluding Abdelmoneim et al | 0.53 | 0.126 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


