Chapter 153
Mesenteric Vascular Disease
Acute Ischemia
Michelle C. Martin, Mark C. Wyers
Acute mesenteric ischemia (AMI) is a life-threatening condition with a well-documented dismal prognosis as a result of catastrophic abdominal events. Klass was the first surgeon to focus on the restoration of arterial blood supply in an attempt to salvage acutely ischemic bowel. He reported the first operative superior mesenteric artery (SMA) embolectomy for AMI in 1951.1 The next 2 decades produced more such reports and increasing success with SMA thromboembolectomy and thromboendarterectomy2 for the treatment of acute SMA thrombosis. Unfortunately, successful outcomes after the treatment of acute thrombotic SMA occlusion remained elusive, with mortality rates of 70% to 90%. Early and liberal use of angiography was championed by Boley3,4 and Clark5,6 and their respective coauthors in the early 1970s. With an aggressive approach, including the use of vasodilators, they demonstrated a reduction in the AMI mortality rate to approximately 50%. This mortality rate has not been reproduced or improved on, despite all the major advances that have taken place in the subsequent 40 years. In fact, more contemporary reviews still report mortality rates between 60% and 80%.7,8 Treatment of acute mesenteric arterial occlusions has traditionally been surgical, with intensive medical support, as well as some limited interventional options for nonocclusive mesenteric ischemia (NOMI). There are, however, increasing reports of endovascular or hybrid endovascular-surgical treatments for all forms of AMI that can be applied to the treatment of well-selected patients.
Incidence and Risk Factors
AMI is uncommon, accounting for less than 1 of every 1000 hospital admissions.9 Women are affected three times as frequently as men are. Patients typically present in their 60s to 70s and often have a number of medical comorbidities. Clinical risk factors may provide some clue as to the specific pathophysiologic process. Patients with a history of atrial fibrillation or flutter, recent myocardial infarction, congestive heart failure, or peripheral arterial emboli are at risk for an SMA embolus. In contrast, a careful history may reveal postprandial abdominal pain, weight loss, and food intolerance, all of which clearly raise the suspicion of an acute SMA thrombotic occlusion. A review of a national database identified that patients undergoing angioplasty and stenting for AMI had higher rates of comorbidities than those undergoing open surgical repair, including hypertension, peripheral vascular disease, coronary artery disease, atrial fibrillation or flutter, and chronic renal failure.10 Finally, a patient with NOMI is likely to be critically ill and to have suffered a significant hemodynamic insult in the preceding hours to days. Cardiac surgery and hemodialysis patients are classically at highest risk for NOMI. The diagnostic algorithm should be tailored on the basis of the suspected cause—arterial embolization, arterial thrombosis, or nonocclusive ischemia.
Pathophysiologic Classification
Arterial Embolism
Arterial embolism is the most common pathophysiologic mechanism of AMI, accounting for 40% to 50% of cases.11 Most embolic events are thromboembolic and arise from a cardiac source. The historical elements that place a patient at risk for a thromboembolic event include atrial tachyarrhythmia, low ejection fraction (congestive heart failure, cardiomyopathy), recent myocardial ischemia or infarction, and ventricular aneurysm. Nearly one third of patients with an SMA embolus have had an antecedent embolic event. There is some speculation, however, that the overall incidence of thromboembolism is declining secondary to better guidelines for and compliance with anticoagulation in patients with atrial fibrillation.12
Although significantly less common than a cardiac source, additional proximal arterial sources of atheroembolic material should also be considered. In these instances, a previous history of cardiac valvular disease, endocarditis, proximal aneurysm, or recent catheter-based angiography may be noted.13
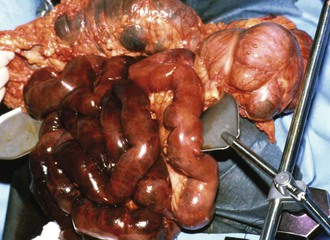
The SMA is the mesenteric vessel most commonly affected by emboli because of its oblique origin from the visceral aortic segment. Thromboemboli tend to lodge in the proximal SMA because of their size, just beyond the first few jejunal branches as the SMA tapers. A minority (15%) may lodge at the SMA origin, but 50% lodge distal to the middle colic artery,14 creating a classic pattern of ischemia that spares the first portion of the small intestine and the transverse colon (Fig. 153-1). Atheroembolic emboli, in contrast, tend to be smaller and therefore lodge in the more distal mesenteric circulation. As a result, these emboli are likely to affect bowel perfusion less often and in more localized areas.
Arterial Thrombosis
Acute arterial thrombosis superimposed on preexisting severe atherosclerotic disease is the next most common cause of AMI, accounting for 25% to 30% of cases.11,15 Many patients with visceral atherosclerotic disease are asymptomatic before a thrombotic event. Autopsy studies have shown that 6% to 10% of the population has a greater than 50% stenosis in at least one mesenteric artery.19 Further, the incidence of asymptomatic celiac artery or SMA stenosis greater than 50% in patients undergoing arteriography for other peripheral vascular disease may be as high as 27%.16 Bowel infarction is more insidious in onset because extensive collaterals are able to maintain viability until there is final closure of a critically stenotic vessel or collateral. Consideration must also be given to how vascular collaterals, interrupted by previous abdominal surgery or bowel resection, may affect the region of bowel involvement. Once established, however, the pattern of infarction is more confluent, unlike that seen with embolic causes. There is no sparing of the proximal jejunum or middle colic distribution because the SMA origin is almost uniformly occluded.17,18
Acute-on-chronic presentations are not uncommon. Endean et al19 suggested that up to 20% of AMI patients have a history of postprandial abdominal pain, food avoidance, or weight loss. Even for patients with asymptomatic mesenteric arterial lesions, the natural history of mesenteric occlusive disease is progressive and potentially morbid. In one report, 86% of patients with more advanced three-vessel mesenteric atherosclerosis experienced vague abdominal discomfort, had frank mesenteric ischemia, or died during a 2.6-year mean follow-up interval.20 Therefore, a history of symptoms of chronic mesenteric ischemia should be elicited.
In addition, complications of mesenteric revascularization with angioplasty and stenting for chronic mesenteric ischemia can lead to AMI. Immediate endovascular failures including distal embolization, vessel perforation, dissection, stent migration, and thrombosis result in higher mortality and morbidity and longer hospital length of stay compared with stent patients without complications.21 The use of antiplatelet therapy reduces the risk of distal embolization or vessel thrombosis perioperatively. All patients who have undergone revascularization for mesenteric ischemia require long-term follow-up as both open and endovascular approaches can lead to the development of AMI secondary to graft or stent thrombosis as a result of intimal hyperplasia, in-stent stenosis, or stent fracture.
Spontaneous visceral artery dissection and acute extension of aortic dissection can also serve as mechanisms for abrupt mesenteric vessel occlusion and thrombosis, as discussed later.
Nonocclusive Mesenteric Ischemia
Mesenteric vasospasm, usually in the distribution of the SMA, is the sine qua non of NOMI. This form of AMI accounts for approximately 20% of presentations and carries the highest mortality rates because of its frequent association with multisystem organ failure.22,23 Initial descriptions were in postmortem observation of small intestinal gangrene in patients who had shown no evidence of arterial or venous occlusive disease.24 Several reports both remotely and recently have described this diagnosis in patients with severe cardiac failure, sometimes but not always with concomitant visceral athererosclerosis.3,14,25–29 These observations formed the basis for the hypothesis that cardiac failure, peripheral hypoxemia, paradoxical splanchnic vasospasm, and reperfusion injury may all contribute to the development of NOMI. Perhaps resulting from excessive sympathetic activity during cardiogenic shock or hypovolemia, the vasospasm represents a homeostatic mechanism that attempts to maintain cardiac and cerebral perfusion at the expense of visceral and peripheral organs. Vasopressin and angiotensin are the likely neurohormonal mediators of this process.30,31 In the current era, vasoactive medications such as epinephrine, norepinephrine, and vasopressin have also been associated with the development of NOMI.29–32 Once mesenteric vasospasm is initiated, it may persist even after correction of the initiating event. Although intestinal autoregulation may initially offset reductions in blood flow, the autoregulatory capacity is exceeded after several hours.28
The exact mechanism of persistence of vasospasm is unknown, but it plays an important role in the development and maintenance of occlusive and nonocclusive mesenteric ischemia as well as in reperfusion phenomena complicating mesenteric revascularization.33 The degree of ischemia-reperfusion injury appears to be related to the frequency as well as the duration of ischemic episodes. Clark and Gewertz34 demonstrated that two 15-minute periods of low flow followed by reperfusion resulted in more severe histologic injury than a single 30-minute period of ischemia. NOMI results in an analogous scenario, whereby hypoperfusion may be partial and occasionally repetitive. Episodic reperfusion is thought to prime the ischemic tissue with leukocytes that are attracted to and produce reactive oxygen species.35 This concept is supported by studies demonstrating attenuation of ischemia-reperfusion injury by reperfusion with leukopenic blood or blockade of endothelial cell surface receptors for leukocyte adherence.36,37 Reports of NOMI after elective mesenteric revascularization further support reperfusion injury in the pathogenesis of NOMI.33
Final Common Pathway of Bowel Ischemia
The degree of reduction in blood flow that the bowel can tolerate without permanent cellular damage is remarkable. Only one fifth of the mesenteric capillaries are open at any given time, and normal oxygen consumption can be maintained with only 20% of maximal blood flow.38 Proposed mechanisms that result in the preservation of splanchnic tissue perfusion include direct arteriolar smooth muscle relaxation and a metabolic response to adenosine and other metabolites of mucosal ischemia.39 In addition, the intestinal mucosa is able to extract increasing amounts of oxygen during hypoperfusion40 to preserve mucosal integrity during periods of metabolic insult. Prolonged ischemia, whatever the pathophysiologic cause, leads to disruption of the intestinal mucosal barrier, primarily through the actions of reactive oxygen metabolites and polymorphonuclear neutrophils.8 The mucosal surfaces are affected first because the metabolic demand of the mucosa is much higher than that of the serosa. Clinically, this may be manifested with malabsorption and heme-positive diarrhea before the onset of other symptoms.
Diagnostic Evaluation
Delays in diagnosis and treatment remain the greatest challenge to reducing morbidity and mortality for all forms of AMI.41 The pathologic causes of abdominal pain and tenderness in the elderly population fluctuate, and all too frequently, AMI is not included in the initial differential diagnosis. Boley demonstrated that in patients with SMA embolism, a condition that usually results in the most rapid clinical decline because of the lack of established collateral circulation, survival decreases from approximately 50% to 30% when the diagnosis is made more than 24 hours after the onset of symptoms.14 Two more recent reviews suggested that only one third of AMI patients were correctly identified before surgical exploration or death.42,43 Bowel necrosis is probably the best indicator that there has been a delay in diagnosis and together with advanced patient age has been linked to higher mortality rates.41 A high index of suspicion in the setting of a compatible history and physical examination serves as a cornerstone of prompt treatment. Once AMI is suspected, the clinician should quickly move to appropriate testing to confirm the diagnosis, keeping in mind that the best first test may be an operation or arteriography.
Recently, there has been a paradigm shift in the diagnostic algorithm for AMI. The older model, as championed by Boley and associates,4,44 advocated early and aggressive use of diagnostic angiography. Today, computed tomographic angiography (CTA) has supplanted diagnostic angiography when occlusive AMI (but not necessarily NOMI) is suspected. With nearly universal 24-hour access to high-resolution CTA, the diagnosis of SMA embolus or thrombotic occlusion can be confirmed, and the bowel can be evaluated concomitantly to support or to refute the diagnosis.
Angiography still plays an important role in the diagnosis and treatment of AMI, however. In fact, with an ever-expanding list of endovascular treatments or adjuncts, its therapeutic role is, in many ways, strengthened. Only its timing and application may be somewhat changed. Gone are the days when arteriography was performed and the patient returned hours later, after the study had been read. Increasingly, vascular surgeon–interventionalists are able to combine the final steps of diagnosis and treatment on one table in the operating room environment. This allows general and vascular surgeons to work together to provide definitive treatment. In a well-designed treatment pathway, this coupling of angiography and definitive surgical treatment can save precious time in the treatment of these challenging patients.
Clinical Presentation
Many of the signs and symptoms of AMI are easily mistaken for other, more common intra-abdominal pathologic processes, such as pancreatitis, cholecystitis, appendicitis, diverticulitis, and bowel obstruction. The classic description of early symptoms is pain that is greater than expected on the basis of physical examination findings. However, depending on the exact cause of AMI and the timing of presentation, this classic presentation may be absent in 20% to 25% of cases.22 Until there is transmural involvement of the bowel, there is relatively little peritoneal irritation and therefore tenderness to palpation may be minimal. For patients who present with SMA embolism, the onset is more abrupt, without a prodrome, with rapid progression. On occasion, as in Klass’ original description, there may be sudden and forceful bowel evacuation shortly after the onset of pain.1 An intermediate rate of progression can be seen with SMA thrombosis because many of these patients have developed collaterals. Their subacute presentation may start weeks before the final acute insult that prompts them to seek medical attention. Patients may have abdominal pain, distention, diarrhea, acidosis, sepsis, or gastrointestinal bleeding, singly or in combination. NOMI patients may have the most insidious onset and a protracted clinical course. Furthermore, they are least able to offer any history because they are already critically ill, frequently in a hospital critical care setting. The pain associated with NOMI can be variable in location as well as in character.
Laboratory Evaluation
The most common laboratory abnormalities are hemoconcentration, leukocytosis, high anion gap, and possibly lactic acidosis in more advanced cases. High amylase, aspartate aminotransferase, and lactate dehydrogenase can also be observed. All these serum markers, however, are insensitive and nonspecific for the diagnosis of mesenteric ischemia.45,46 D-dimer elevation after 2 hours has been correlated with the presence of AMI in a rat model.47 Similarly, Acosta et al42 showed elevated D-dimer levels in AMI patients who presented within 24 hours of the onset of symptoms, in contrast to patients with inflammatory conditions or bowel obstruction. No patient with a D-dimer concentration of 0.3 µg/mL (1.6 nmol/L) or less had acute SMA occlusion, suggesting this as a possible exclusionary test.42 In recent investigations, urinary fatty acid–binding protein (FABP) levels hold promise as a diagnostic test for AMI.48 First discovered in rodents, the human homologue was found to be elevated in cases of documented bowel infarction.49,50 Thuijls et al48 demonstrated elevated plasma and especially urinary levels of intestinal FABP, liver FABP, and ileal bile acid–binding protein in patients diagnosed with intestinal ischemia. Fifty consecutive patients suspected of AMI had levels taken before diagnosis. In all 22 patients with confirmed AMI, all three levels were elevated. Furthermore, the urinary concentration of ileal bile acid–binding protein was elevated specifically when the ileum was involved, allowing localization. Overall, the test was rapid and potentially useful for early diagnosis of intestinal ischemia.
Diagnostic Imaging
Abdominal Plain Radiography
Abdominal plain radiographs are normal in up to 25% of patients with AMI.51 However, ileus may be an early finding consistent with mesenteric ischemia, and advanced cases of intestinal ischemia may show evidence of bowel wall edema (“thumbprinting”) or pneumatosis. Plain abdominal radiography is most useful in excluding other causes of abdominal pain, such as bowel obstruction or perforation.
Duplex Ultrasonography
Duplex ultrasonography accurately identifies high-grade stenoses of the celiac artery and SMA. It is the noninvasive diagnostic study of choice in patients with symptoms suggesting chronic mesenteric ischemia but has little or no role in the diagnosis of AMI for several important reasons. It is highly technologist dependent, and many hospitals do not have ready access to such specialized vascular laboratory testing, especially during off-hours. The presence of intestinal gas—which is the rule in any nonfasting patient, let alone someone suspected of having AMI—can easily obscure visualization of the mesenteric vessels. Any abdominal tenderness also compromises the study and adds to the patient’s suffering.
Computed Tomography
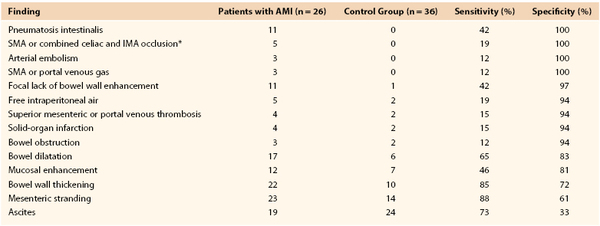
Several authors have described the use of ultrafast multidetector CTA (MDCTA) for the evaluation of both acute and chronic mesenteric ischemia.52–55 The widespread availability of the newest generation of computed tomography (CT) scanners has evolved the diagnostic algorithm for AMI and offers greater speed than angiography. CTA provides a significant amount of information about the central arterial and venous circulations. Accurate timing of contrast injection and fine slices through the upper abdomen usually provide excellent visualization of the celiac artery and SMA (Figs. 153-2 and 153-3). CT can also exclude other causes of abdominal pain and assess bowel perfusion to some extent. The timing of intravenous contrast administration is tailored to the specific clinical question. The use of traditional oral “positive” contrast agents detracts from image quality, and most visceral CTA protocols recommend the use of a “negative” oral contrast agent such as water (500 to 750 mL) given immediately before the scan. This prevents image artifact from pooled areas of high opacification within the intestinal tract and also enhances the ability to see bowel wall enhancement (or the lack thereof) in the late arterial phase of the arterial contrast bolus.51 Main branches of the celiac artery and SMA are seen remarkably well by use of MDCTA because of thinner collimation (0.5 to 1.5 mm) and overlapping data acquisition. This reduces the amount of volume averaging and creates higher quality three-dimensional volume sets for reformatting and interpretation.
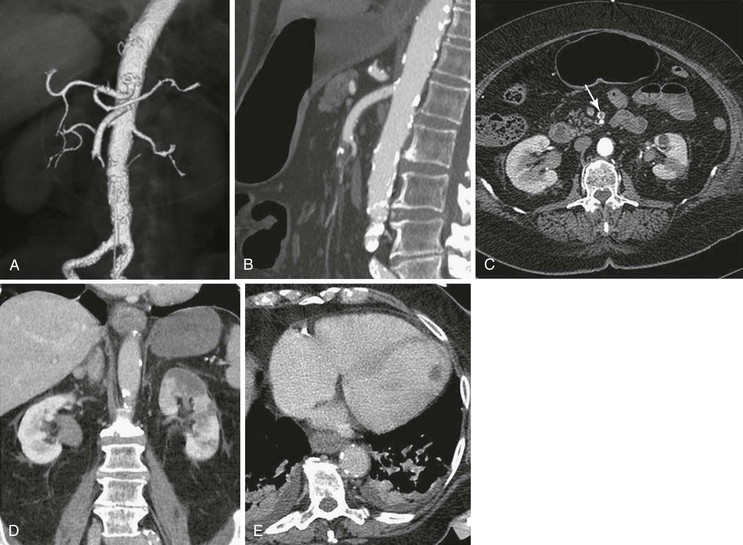
Figure 153-2 A, Three-dimensional volume rendering of arterial phase MDCTA shows an abrupt mid–superior mesenteric artery (SMA) occlusion consistent with embolus. B, Sagittal multiplanar reformat shows the same occlusion. C, SMA occlusion seen on an axial CTA slice (arrow). D, Coronal multiplanar reformat reveals left kidney infarction. E, Transverse portal venous phase MDCTA depicts thrombus in the left ventricle as the likely embolic source. (From Aschoff AJ, et al: Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging 34:345-357, 2009.)
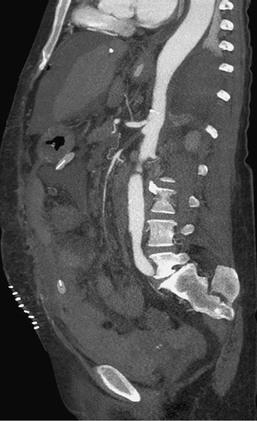
Figure 153-3 CTA sagittal multiplanar reformat shows an acute thrombotic occlusion of the proximal superior mesenteric artery. Bowel resection was performed 3 days before this image was obtained. The concomitant celiac stenosis suggests an acute-on-chronic presentation of acute mesenteric ischemia. (From Aschoff AJ, et al: Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging 34:345-357, 2009.)
Initial interest in so-called biphasic CT was generated in the evaluation of pancreatic and hepatic lesions; it includes an arterial phase and a delayed phase, allowing time-based visualization of the portal venous system. This method has been applied more recently to detect the early findings of AMI. That is, the same scan is used to detect arterial narrowings or occlusions and to assess associated changes in bowel wall thickness, pneumatosis, and mucosal or bowel wall enhancement patterns that support the diagnosis of AMI and can also diagnose mesenteric venous thrombosis (Fig. 153-4). Kirkpatrick et al56 sought to improve on a previous retrospective report that used single-detector helical CT.57 Sixty-two patients suspected of having AMI underwent biphasic MDCTA and were evaluated prospectively. As in the previous study, no single CT finding was both sensitive and specific (Table 153-1). Twenty-six patients, however, had AMI confirmed at surgical exploration or on pathologic examination, and all these patients had been correctly identified by the interpreting radiologist as having AMI. An additional four scans interpreted as demonstrating AMI turned out to be false positives, with the ultimate diagnosis of Crohn’s disease (2), neutropenic enterocolitis (1), and infectious enterocolitis (1). Thus, the initial interpretation had a sensitivity of 100% and a specificity of 89% for the diagnosis of AMI. In the same study, CTA visualization was judged to be satisfactory in all cases up to second-order branches of both the celiac artery and the SMA. Angiography was available in only three patients but correlated well with the CTA findings. In a more recent retrospective chart review using similar MDCTA protocols and even higher resolution CT, Aschoff et al58 were able to duplicate Kirkpatrick’s findings in terms of overall accuracy. Again, no one CT finding was perfectly sensitive or specific for AMI, but using a combination of CT criteria (pneumatosis, bowel edema, other solid-organ infarction) to generate an overall impression, these authors were able to achieve positive and negative predictive values of 100% and 96%, respectively.
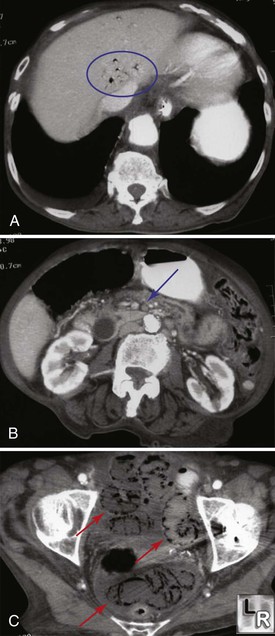
Figure 153-4 A, CTA demonstrating hepatic venous air (circle). B, The superior mesenteric artery is occluded (arrow). C, There is extensive colonic pneumatosis and ascites (arrows). (From http://www.learningradiology.com/notes/ginotes/mesentericischemiapage.htm.)
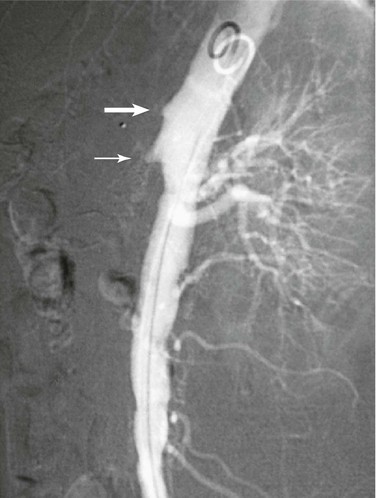
Arteriography
Traditional catheter-based arteriography (Fig. 153-5) has been supplanted by MDCTA as the definitive diagnostic study for occlusive forms of AMI. Patients with strongly suspected NOMI may still benefit from a more traditional angiographic approach, as long as there is no immediate need for exploration based on the physical examination. Arteriography’s role in the therapy for all forms of AMI, however, has been strengthened. It offers several complementary or stand-alone treatment options, depending on the specific disease, including injection of intra-arterial vasodilators,59 thrombolysis,60 and angioplasty with or without stenting.61 These are discussed in more detail in the sections on treatment of nonocclusive mesenteric ischemia and endovascular treatment. As vascular surgeons become accomplished interventionalists and as intraoperative fluoroscopy improves, the confirmatory diagnostic arteriogram can be accomplished in the operating room, followed by immediate revascularization and surgical exploration and thus limiting a delay to treatment.
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) of the splanchnic vessels is theoretically appealing because it is noninvasive, avoids the risk of allergic reaction and nephrotoxicity associated with iodinated contrast agents, and may not be as operator dependent as duplex ultrasound. Magnetic resonance imaging of the mesenteric vasculature can incorporate both functional and anatomic evaluations, which hold promise for the diagnosis of chronic mesenteric ischemia.62,63
The weakness of MRA is its relatively poor spatial resolution, which, even on the best systems, is limited to 1 mm3. Gadolinium-enhanced MRA does not currently provide sufficient resolution to evaluate distal braches or the inferior mesenteric artery or to demonstrate distal emboli, nonocclusive low-flow states, small-vessel occlusion, or vasculitis.64 This essentially limits evaluation of the mesenteric arteries to the proximal celiac artery and SMA. In addition, the secondary signs of AMI, such as indurated fat and bowel wall thickening, which are routinely delineated by CT, are more difficult to assess with MRA. In essence, these issues as well as the lack of uniform and timely availability and difficulty in obtaining interpretation make MRA a less reliable modality.
Diagnostic Laparoscopy
Laparoscopy in the setting of AMI has limited ability to assess bowel viability. Serosal color can be difficult to judge and can be distorted significantly by a malfunctioning or improperly calibrated camera. Furthermore, segmental ischemia can be missed because of the difficulty in “running” the bowel along its entire length and over all surfaces. To increase the sensitivity of diagnostic laparoscopy, some authors have successfully used fluorescein with a laparoscopic ultraviolet light.65–67 Nevertheless, diagnostic laparoscopy for this indication has not been widely accepted68 because it may still miss areas of nonviable bowel.
Treatment
Initial Resuscitation and Critical Care
Fluid resuscitation of a patient with AMI should begin immediately with isotonic crystalloid solution and continue with blood, if necessary. Electrolyte imbalances (hyperkalemia) should be monitored and corrected. Invasive monitoring (hourly urine output, continuous central pressure, and arterial pressure monitoring) is advisable from the beginning to ensure that all parameters are optimized before intravenous contrast administration or operative exploration. Broad-spectrum antibiotics should be given to guard against translocated bacteria and sepsis. If there are no contraindications, intravenous heparin should also be administered to maintain a partial thromboplastin time greater than twice normal.
The presentation of sepsis and organ dysfunction in these patients is common, and for the most part, these disorders are managed as they would be in other situations. Vasopressors, however, may worsen ischemia in marginally viable bowel and exacerbate visceral vasospasm. Before the initiation of any vasopressor, volume resuscitation must be confirmed by the presence of adequate right-sided heart filling pressures. Because of the large and ongoing fluid sequestration in these patients, 24-hour crystalloid requirements in excess of 15 L are not uncommon. When necessary, better vasopressor options include low- to mid-dose dopamine (3 to 8 µg/kg/min) and epinephrine (0.05 to 0.10 µg/kg/min). Pure α-adrenergic agents should be avoided if possible, even after successful revascularization.8
Treatment of Nonocclusive Mesenteric Ischemia
NOMI accounts for more than 10% to 20% of cases of acute mesenteric circulatory disorders and leads to extensive irreversible intestinal necrosis. The prognosis is poor, despite the absence of organic obstruction in the principal arteries. The primary treatment of NOMI is medical, with extensive critical care support and prompt arteriography. Operative exploration is reserved for signs of peritonitis suggesting the presence of gangrenous bowel that requires excision.
Interventional therapies can be initiated at the time of the diagnostic arteriogram and are targeted at relieving vasospasm by intra-arterial infusions of vasodilator medications (Fig. 153-6). The angiographic appearance of NOMI can be subtle, but Siegelman et al69 described four reliable arteriographic criteria for the diagnosis of mesenteric vasospasm: (1) narrowing of the origins of multiple branches of the SMA, (2) alternate dilatation and narrowing of the intestinal branches—the “string of sausages” sign (Fig. 153-7), (3) spasm of the mesenteric arcades, and (4) impaired filling of the intramural vessels. The most common intra-arterial agent used in the majority of reports is the phosphodiesterase inhibitor papaverine. There are multiple case reports of the local infusion of vasodilators—either papaverine18,70,71 or prostaglandin analogues72—leading to improvement.
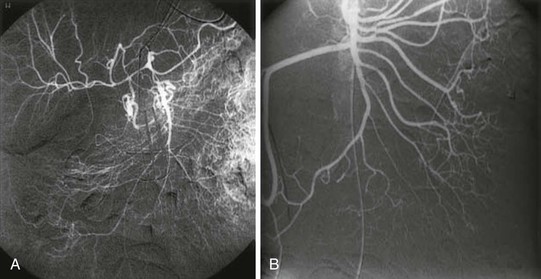
Figure 153-6 Arteriogram with selective superior mesenteric artery (SMA) injections. A, Severe SMA vasospasm with narrowing of all SMA branches. Intramural vessels are not seen owing to severe ischemia. B, There is significant improvement after several hours of vasodilator administration. (From Trompeter M: Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol 12:1179-1187, 2002.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree